Celltrion’s Remsima on course to become Korea’s 1st blockbuster drug
It aims to offer the biosimilar for intravenous and subcutaneous injections, as well as antibody-drug conjugates
By Nov 25, 2024 (Gmt+09:00)
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


Celltrion Inc.'s Remsima, the world’s first biosimilar monoclonal antibody, is on track to exceed 1 trillion won ($713 million) in sales this year to become the first Korean drug to do so.
Remsima raked in 979.7 billion won in sales in the first three quarters ended in September, according to Celltrion on Monday. Based on these three quarters, its sales are expected to reach about 1.2 trillion won for all of 2024.
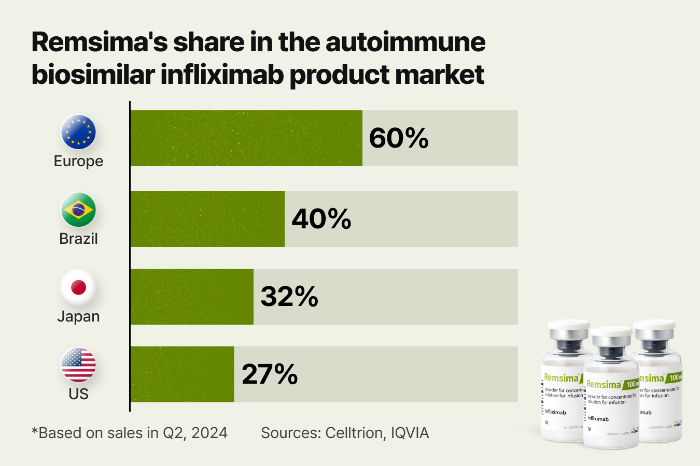
Remsima, the first biosimilar to Janssen’s Remicade, was approved by Europe as the world's first antibody biosimilar in 2013 and got the nod from the US Food and Drug Administration (FDA) in 2016. The drug was credited with spurring growth in the biosimilar market. Currently, it sells in more than 100 countries.
Remsima is available in both vein infusion and subcutaneous injection forms, called Remsima IV and Remsima SC, respectively. Celltrion is also developing the medication as an antibody-drug conjugate type.
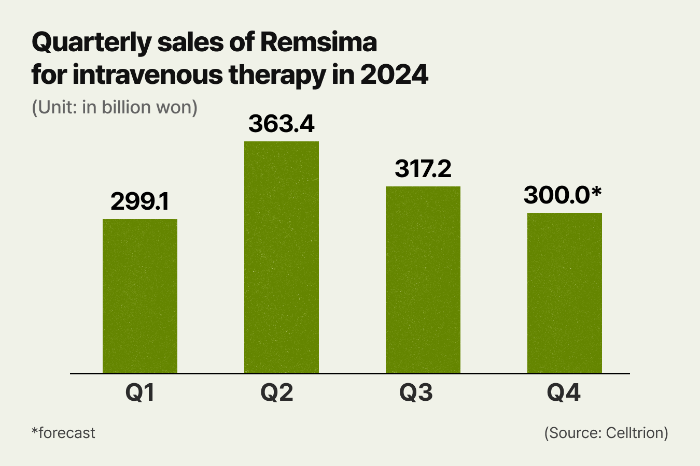
Remsima is an anti-inflammatory medicine that contains the active substance infliximab. It is much cheaper than Remicade, selling at about one-fifth the price of the original drug with the same efficacy. It is prescribed to patients with intractable chronic diseases such as rheumatoid arthritis for long-term treatment.
Remsima SC, launched in 2020, is the world’s first SC infliximab medication referencing Remicade.
Celltrion aims to bring its total biosimilar offerings to 11 by 2025 and 22 by 2030, a significant increase from its current roster of six biosimilars.
Another Korean blockbuster drug candidate is Yuhan Corp's lung cancer treatment Lazertinib.
The first antibiotic drug developed by a South Korean company secured approval from the FDA earlier this year as a combination therapy with a Johnson & Johnson antibiotic.
It is expected to join the ranks of some 150 global blockbuster drugs by 2027 when the company forecasts its sales to surpass 1 trillion won a year.
Write to Jeong Min Nam at peux@hankyung.com
Yeonhee Kim edited this article.
-
 Bio & PharmaCelltrion earmarks billions of dollars to build CDMO plant: chairman
Bio & PharmaCelltrion earmarks billions of dollars to build CDMO plant: chairmanSep 11, 2024 (Gmt+09:00)
2 Min read -
 Bio & PharmaCelltrion eyes M&A in Europe, $3.3 bn Zymfentra sales: chairman
Bio & PharmaCelltrion eyes M&A in Europe, $3.3 bn Zymfentra sales: chairmanMay 23, 2024 (Gmt+09:00)
4 Min read -
-
-
-
 Bio & PharmaCelltrion Healthcare's Remsima SC to be launched in US in 2024
Bio & PharmaCelltrion Healthcare's Remsima SC to be launched in US in 2024Nov 30, 2023 (Gmt+09:00)
1 Min read -
-
 Bio & PharmaCelltrion Healthcare wins Remsima order in France, Italy
Bio & PharmaCelltrion Healthcare wins Remsima order in France, ItalyOct 18, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion’s Remsima SC praised in Europe as preferred biosimilar
Bio & PharmaCelltrion’s Remsima SC praised in Europe as preferred biosimilarJun 02, 2023 (Gmt+09:00)
2 Min read -
-
 Bio & PharmaCelltrion increases its stake in British ADC developer Iksuda
Bio & PharmaCelltrion increases its stake in British ADC developer IksudaJan 25, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion's Remsima receives approval in 100 countries in 10 years
Bio & PharmaCelltrion's Remsima receives approval in 100 countries in 10 yearsJan 03, 2023 (Gmt+09:00)
1 Min read


