Bio & Pharma
Celltrion releases Remsima SC in Denmark
The company won the Danish government's tender for autoimmune disease treatments and will supply until 2025
By Apr 19, 2024 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


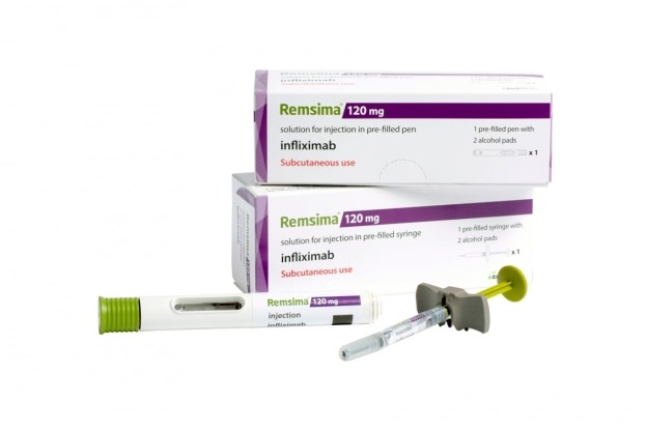
South Korea's Celltrion Inc. announced on Thursday that it released its autoimmune disease treatment Remsima SC (infliximab) in Denmark.
Remsima SC is a subcutaneous (SC) formulation of the autoimmune disease treatment Remsima, containing the ingredient infliximab.
It obtained a new drug approval from the US Food and Drug Administration (FDA) and a biobetter from European Medicine Agency (EMA).
The company started to supply Remsima SC to the Norwegian market in February.
The Danish government created a national tender specifically for the SC form of infliximab and Celltrion secured this tender as the sole supplier, facing no competition.
Remsima SC will be distributed directly by Celltrion's Danish subsidiary, with the initial sales phase planned to last one year starting this month.
In anticipation of growing demand, the subsidiary is ramping up its marketing efforts to increase prescription rates.
The company plans to expand its local workforce by the end of the year, enhancing its direct sales capabilities and strengthening its foothold in the European market.
Write to Jeong Min Nam at peux@hankyung.com
More to Read
-
-
 Bio & PharmaCelltrion Healthcare's Remsima SC to be launched in US in 2024
Bio & PharmaCelltrion Healthcare's Remsima SC to be launched in US in 2024Nov 30, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion to supply Remsima to Brazil gov’t in three-year row
Bio & PharmaCelltrion to supply Remsima to Brazil gov’t in three-year rowNov 10, 2023 (Gmt+09:00)
1 Min read -
Comment 0
LOG IN


