Bio & Pharma
Celltrion’s Remsima SC praised in Europe as preferred biosimilar
The subcutaneous injection type is well-received in Europe for its relative convenience and easy administration
By Jun 02, 2023 (Gmt+09:00)
2
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


MILAN – South Korea's biopharmaceutical company Celltrion Inc. is known in Europe for its biosimilar products such as autoimmune disease treatment Remsima; blood cancer treatment Truxima; and breast cancer medication Herzuma.
Referencing Janssen’s Remicade, an anti-inflammatory medication for immune-system diseases, Celltrion’s Remsima holds more than half the European market for such a biosimilar, also known as infliximab, its active ingredient.
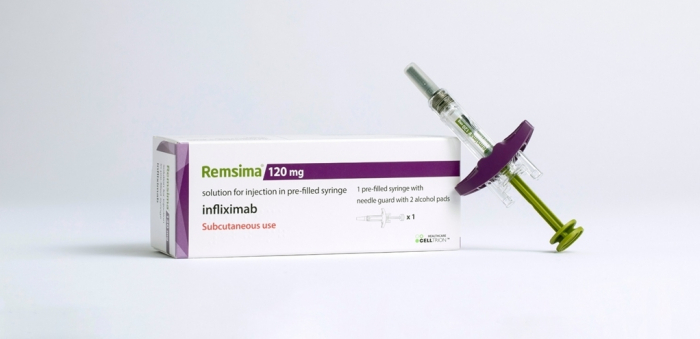
With its intravenous injection type Remsima IV already among the top-selling infliximab products in the region, Remsima SC, a subcutaneous injection type, is also rapidly becoming a sought-after biosimilar for its convenience.
Remsima SC is the world’s first and only infliximab available as a subcutaneous injection to treat autoimmune diseases such as rheumatoid arthritis, ankylosing spondylitis, ulcerative colitis, Crohn's disease and psoriasis.
“With the previous IV formulation, patients needed to visit the hospital every three months to assess their condition. After switching to SC, however, if patients are fine, they only need to come once a year,” said Dr. Raj Sengupta of the UK’s Royal United Hospitals Bath.
Speaking at a European Congress of Rheumatology (EULAR) symposium hosted by Celltrion under the theme “Infliximab IV to SC - Mainstay Therapy in Rheumatology,” he said Remsima SC demonstrated superiority over IV-type infliximab in clinical tests for patients with rheumatoid arthritis and ankylosing spondylitis.
The rheumatologist said he even recommends the SC type to patients on their first visit.
LONGER-LASTING EFFICACY
Another speaker, Dr. Roberto Giacomelli of Italy’s Policlinico Universitario Campus Bio-Medico, said Remsima SC showed longer-lasting efficacy on rheumatism patients compared with those treated with IV-type infliximab.
“Switching from the intravenous to the subcutaneous injection type means lower costs from a pharmacological perspective,” he said.
Celltrion said in January that Remsima, a monoclonal antibody biosimilar, has been approved in more than 100 countries globally.
Last month, Celltrion Healthcare Co., the Korean biosimilar maker’s global marketing arm, said it is also launching Remsima SC in Brazil, the largest pharmaceutical market in Latin America.
The company received Remsima approval from Europe’s drug agency in 2013 and the US Food and Drug Administration in 2016.
RAPID GROWTH IN EUROPE
Thanks to positive feedback from local medical professionals, Remsima SC is posting rapid growth in Europe.

Remsima SC’s European market share rose to 16% in the fourth quarter of last year, up from a mere 1% in the second quarter of 2020, when it was launched there.
Combined with the IV type, Remsima’s total European market share reached 60.6% in the fourth quarter.
The biosimilar has been the most-prescribed infliximab in Europe since it overtook the original, Remicade, in 2017.
Biosimilars are products that have been demonstrated to be similar in efficacy and safety to the originator’s reference product, with the advantages of cost savings and promoting sustainable access to therapies.
Write to Yoo-Rim Kim at youforest@hankyung.com
In-Soo Nam edited this article.
More to Read
-
-
 Bio & PharmaCelltrion's Remsima receives approval in 100 countries in 10 years
Bio & PharmaCelltrion's Remsima receives approval in 100 countries in 10 yearsJan 03, 2023 (Gmt+09:00)
1 Min read -
 EarningsCelltrion's Q3 earnings boosted by Remsima sales in Europe
EarningsCelltrion's Q3 earnings boosted by Remsima sales in EuropeNov 09, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion gains sales approval for biosimilar Vegzelma in Europe
Bio & PharmaCelltrion gains sales approval for biosimilar Vegzelma in EuropeAug 19, 2022 (Gmt+09:00)
2 Min read -
 Bio & PharmaCelltrion to sell biosimilar products via its own channel in Europe
Bio & PharmaCelltrion to sell biosimilar products via its own channel in EuropeMay 11, 2022 (Gmt+09:00)
2 Min read -
 BiotechCelltrion looks beyond biosimilars to develop its own mRNA drugs
BiotechCelltrion looks beyond biosimilars to develop its own mRNA drugsNov 16, 2021 (Gmt+09:00)
4 Min read
Comment 0
LOG IN


