Bio & Pharma
Celltrion Healthcare to supply Yuflyma to five regional gov’ts in Italy
The company plans to provide the biosimilar of Humira to Campania, Umbria, Piedmont, Molise, and Valle d'Aosta
By Sep 18, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Mirae Asset to be named Korea Post’s core real estate fund operator


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


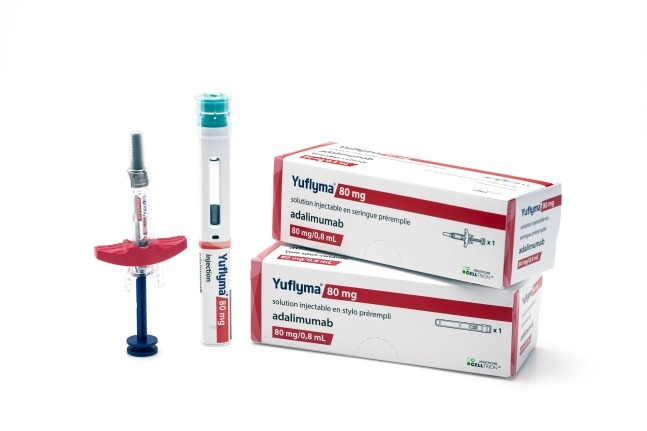
South Korea's Celltrion Healthcare Co. announced on Monday that it will supply its biosimilar Yuflyma (active ingredient: adalimumab) for the treatment of autoimmune diseases to five regions in Italy.
Celltrion Healthcare participated in the bidding held in the third quarter of this year by the regional governments of Campania, Umbria, Piedmont, Molise and Valle d'Aosta in Italy and won the bid. These five regional governments account for approximately 20% of the Italian adalimumab market. Yuflyma supply has already begun in some regions from last month, and it is expected to continue for the next one to three years.
Previously, Yuflyma also won bids for adalimumab in the Emilia-Romagna and Veneto regions of Italy in the first quarter of this year. It is the only high-concentration biosimilar sold in an 80 mg dosage, which provides a competitive advantage, according to the company.
Yuflyma is a biosimilar of the blockbuster biopharmaceutical Humira by the US pharmaceutical company AbbVie Inc. It is used to treat conditions such as rheumatoid arthritis, inflammatory bowel disease, psoriasis, juvenile idiopathic arthritis, and Crohn's disease.
Celltrion Healthcare explained that with expanded prescriptions in Europe, Yuflyma has recorded sales of 54 billion won ($40.7 million) in the first half of this year.
Write to Jeong Min Nam at peux@hankyung.com
More to Read
-
 Bio & PharmaCelltrion Healthcare launches Humira biosimilar Yuflyma in US
Bio & PharmaCelltrion Healthcare launches Humira biosimilar Yuflyma in USJul 04, 2023 (Gmt+09:00)
1 Min read -
-
 Bio & PharmaCelltrion Healthcare enters top 10 in hospital prescriptions in Turkey
Bio & PharmaCelltrion Healthcare enters top 10 in hospital prescriptions in TurkeyApr 21, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaS.Korea's Celltrion Healthcare launches Vegzelma in US
Bio & PharmaS.Korea's Celltrion Healthcare launches Vegzelma in USApr 17, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion Healthcare launches anti-cancer biosimilar Vegzelma in Japan
Bio & PharmaCelltrion Healthcare launches anti-cancer biosimilar Vegzelma in JapanJan 26, 2023 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


