Bio & Pharma
S.Korea's Celltrion Healthcare launches Vegzelma in US
In its first direct sale of medicine on the American market, the company is recruiting sales experts and expanding operations
By Apr 17, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


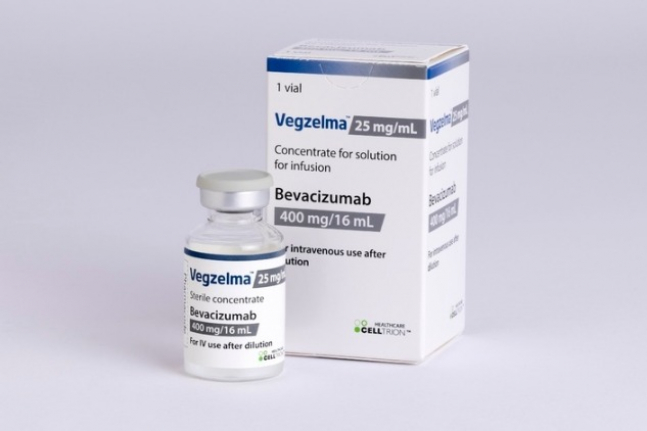
South Korea's Celltrion Healthcare Co. on Monday said it launched on the US market Vegzelma, a biosimilar of Bevacizumab, a medication for colorectal and breast cancer sold under the name Avastin.
The company has prepared for the launch since September last year, when it received approval to sell Vegzelma from the US Food and Drug Administration.
Celltrion Healthcare said it completed inclusion of Vegzelma on the list of drugs reimbursable by public health insurance from the US federal government and sent its first shipment to major wholesalers.
In addition, the company is focused on expanding human resources in the US as Vegzelma is its first drug sold directly in the country. It hired Thomas Nusbickel, chief commercial officer of its incorporated company in America who previously worked for global pharmaceutical giants like Amgen and Pfizer.
"We plan to launch products sequentially in the US ranging from Uplima (active ingredient Adalimumab), which is scheduled for release in this year's second half, to Remsima SC, which is undergoing the approval process for a new drug," Celltrion Healthcare CEO Kim Hyoung-ki said.
Write to Jeong Min Nam at peux@hankyung.com
More to Read
-
 Bio & PharmaCelltrion Healthcare launches anti-cancer biosimilar Vegzelma in Japan
Bio & PharmaCelltrion Healthcare launches anti-cancer biosimilar Vegzelma in JapanJan 26, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion Healthcare lands biosimilar deal in Brazil for 2nd straight year
Bio & PharmaCelltrion Healthcare lands biosimilar deal in Brazil for 2nd straight yearJan 11, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion Healthcare logs record-high Q2 revenue on back of Remsima
Bio & PharmaCelltrion Healthcare logs record-high Q2 revenue on back of RemsimaAug 17, 2022 (Gmt+09:00)
2 Min read
Comment 0
LOG IN


