Celltrion Healthcare launches Humira biosimilar Yuflyma in US
The item is 5% cheaper than its original and boasts a high-concentration and citrate-free formula
By Jul 04, 2023 (Gmt+09:00)
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


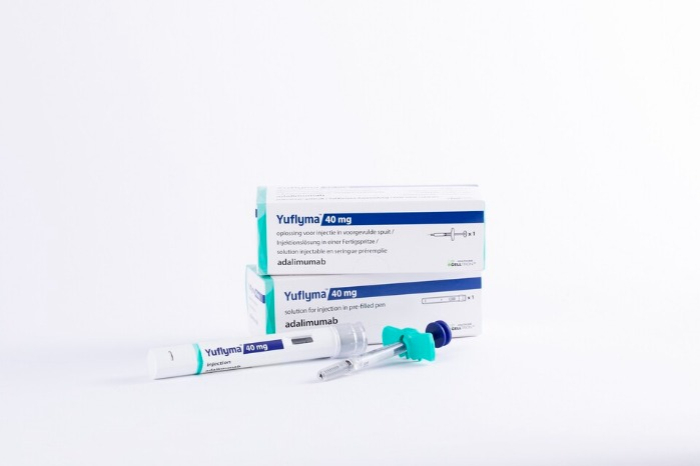
South Korean biopharmaceutical company Celltrion Healthcare on Monday said it launched the day before on the US market Yuflyma, a biosimilar of the autoimmune disease treatment Humira, with the former's active ingredient being adalimumab-aaty.
Humira is a blockbuster drug with global sales last year of $21.2 billion (27.4 trillion won), 87% or $18.6 billion of which came from the US. Available in autoinjector and prefilled syringe form, the treatment has secured indications in America for eight diseases including rheumatoid arthritis, Crohn’s disease and ulcerative colitis.
Celltrion Healthcare set the wholesale acquisition cost of Yuflyma at $6,576.5 per two doses, 5% cheaper than Humira. The company is negotiating with many prescription benefit management companies under a goal of entering the American insurance market, which covers 40% of the country's population.
A high-concentration medicine of 100 milligrams per milliliter that halves dosage from its low-concentration counterparts, Yuflyma has no citrate, which can cause pain. In addition, it can be safely used for up to 30 days if stored at room temperature of 25 degrees Celsius, boasting a shelf life over twice as long as Humira's.
The removal of latex from the biosimilar also prevents allergies to ensure patient convenience and safety.
Celltrion Healthcare is conducting Phase 3 clinical trials worldwide to secure Yuflyma's interchangeability with original treatments in the US and Europe, and completion is slated for the end of next year. The company will also expand prescriptions for the biosimilar by raising its competitiveness.
From Monday, the company's American subsidiary Celltrion USA will launch for patients and medical personnel the Celltrion Connect Patient Support Program, which supports copays and products for uninsured patients or those with insufficient coverage if they meet the conditions.
Write to Ye-Na Kim at yena@hankyung.com
-
 Bio & PharmaCelltrion to develop Humira biosimilar oral medication with US firm
Bio & PharmaCelltrion to develop Humira biosimilar oral medication with US firmJun 05, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion gets FDA approval for Humira biosimilar CT-P17
Bio & PharmaCelltrion gets FDA approval for Humira biosimilar CT-P17May 24, 2023 (Gmt+09:00)
1 Min read -
-
 Bio & PharmaCelltrion Healthcare enters top 10 in hospital prescriptions in Turkey
Bio & PharmaCelltrion Healthcare enters top 10 in hospital prescriptions in TurkeyApr 21, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaS.Korea's Celltrion Healthcare launches Vegzelma in US
Bio & PharmaS.Korea's Celltrion Healthcare launches Vegzelma in USApr 17, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion Healthcare launches anti-cancer biosimilar Vegzelma in Japan
Bio & PharmaCelltrion Healthcare launches anti-cancer biosimilar Vegzelma in JapanJan 26, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion Healthcare lands biosimilar deal in Brazil for 2nd straight year
Bio & PharmaCelltrion Healthcare lands biosimilar deal in Brazil for 2nd straight yearJan 11, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion Healthcare logs record-high Q2 revenue on back of Remsima
Bio & PharmaCelltrion Healthcare logs record-high Q2 revenue on back of RemsimaAug 17, 2022 (Gmt+09:00)
2 Min read


