Bio & Pharma
Samsung Bioepis leads in Soliris biosimilar market in Europe
The S.Korean biosimilar developer's Epysqli takes leadership of the eculizumab biosimilar market in Europe
By May 27, 2024 (Gmt+09:00)
3
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator



STOCKHOLM – Samsung Bioepis Co.'s biosimilar to Soliris for the treatment of patients with rare, life-threatening disease paroxysmal nocturnal hemoglobinuria (PNH) is gearing up to overtake the original eculizumab drug after its successful launch.
The biopharmaceutical development unit under South Korea’s Samsung Group unveiled at the 61st ERA Congress held in Stockholm between May 23 and 26 that its PNH treatment Epysqli commands the large share in the Soliris biosimilar market in Europe less than one year after its launch.
PNH is a rare, life-threatening blood disorder, and Soliris, or eculizumab, by Alexion Pharmaceuticals Inc. is the most widely used original drug to treat PNH and atypical hemolytic uremic syndrome (aHUS) across the world.
Because PNH occurs at a rate of 15 cases per million people, the number of patients is relatively small, making it challenging for biosimilar firms to recruit patients for clinical trials and venture into the business.
Samsung Bioepis and Amgen Inc. are the only pharma developers that have succeeded in developing eculizumab biosimilars.
Epysqli by Samsung Bioepis got a nod for marketing from the European Commission in May last year as a biosimilar to Soliris for the treatment of PNH. The Korean company began its direct sale in the region two months later.
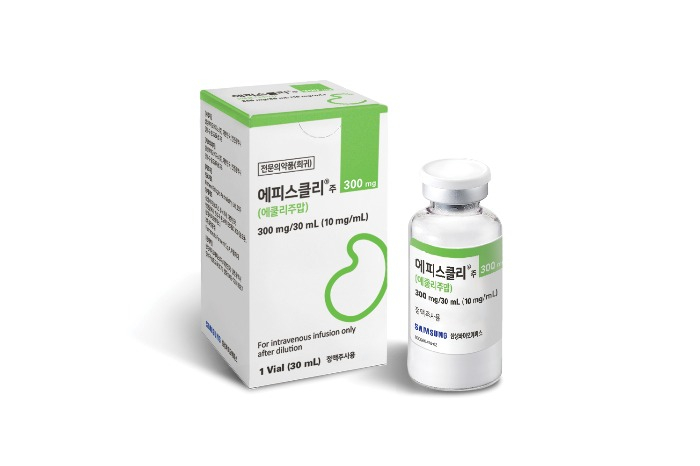
Considering Soliris sales stood at $805 million in Europe in 2023, its market share was estimated to have fallen to the 70 percent range since the launch of its copy drugs, while Samsung Bioepis is estimated to have reaped tens of billions of won from direct sales of Epysqli.
It was the Korean company’s first product sold through direct marketing channels in Europe.
Samsung Bioepis’ Head of Europe Antonio Rito cited the company’s direct marketing as one of Epysqli’s success factors in Europe, saying that marketing capability is as important as drug development and production capabilities to leap to become a big pharmaceutical company.
He said Samsung Bioepis has ascended to a “market leader” in one year, paving the way for direct marketing of the company’s other products.
HIGH-QUALITY PRODUCT POISED TO DEEPEN REACH
Besides its marketing advantage, the Korean eculizumab biosimilar has also gained the similarity in efficacy and safety compared to its reference product.
Epysqli does not contain sorbitol, an ingredient known to cause allergic reactions in hereditary fructose intolerance patients.
Because it is impossible to diagnose babies under two years old with the disorder, doctors prescribe medicines without sorbitol to children.
Of all aHUS patients, about 20% are children.
Epysqli controls the largest share in Germany, Italy and France. It is sold via a group purchasing organization in France and won over 90% of tenders across Italy.
Rito said Samsung Bioepis aims to win 95% of PNH patients and 80% of aHUS patients who are prescribed with Soliris this year so it can become the No. 1 brand in the global rare disease treatment market.
Especially, the global rare blood disease biosimilar market was estimated at 32 trillion won ($23.5 billion) as of mid-2023.

The company is also seeking to expand Epysqli in treating patients with neuromyelitis optical spectrum disorder.
Soliris is already widely used to treat neuromyelitis optical spectrum disorder and generalized myasthenia gravis.
The original eculizumab drug obtained US Food and Drug Administration approval in 2007 and has since posted billions of dollars in sales globally.
Its original developer Alexion was taken over by AstraZeneca plc, a British-Swedish multinational pharmaceutical and biotechnology company, in 2021.
Samsung Bioepis spent eight years developing and completing clinical trials of Epysqli, which is offered to PNH patients as a reasonable alternative to Soliris, which costs between 400 million and 500 million won per patient a year.
Write to Jeong Min Nam at peux@hankyung.com
Sookyung Seo edited this article.
More to Read
-
 Bio & PharmaSamsung Bioepis releases Soliris biosimilar in Europe
Bio & PharmaSamsung Bioepis releases Soliris biosimilar in EuropeOct 19, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSamsung Bioepis to enhance biosimilar presence with Epysqli
Bio & PharmaSamsung Bioepis to enhance biosimilar presence with EpysqliJun 12, 2023 (Gmt+09:00)
3 Min read -
 Bio & PharmaSamsung Bioepis’ SB12 shows same clinical effects as Alexion’s Soliris
Bio & PharmaSamsung Bioepis’ SB12 shows same clinical effects as Alexion’s SolirisJun 14, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaSamsung Bioepis gets approval to sell biosimilar in Europe
Bio & PharmaSamsung Bioepis gets approval to sell biosimilar in EuropeMay 31, 2023 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


