Bio & Pharma
Samsung Bioepis to enhance biosimilar presence with Epysqli
With 10 biosimilars in the pipeline, the Korean company is aggressively targeting the US and European markets
By Jun 12, 2023 (Gmt+09:00)
3
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator



FRANKFURT – Samsung Bioepis Co., a biopharmaceutical unit of South Korea’s top conglomerate Samsung, is looking to gain ground in the rare blood disease biosimilar segment, of which the global market is estimated at 32 trillion won ($24.8 billion).
The Korean biosimilar maker said it is getting positive response from the medical community in Europe for its latest product Epysqli, which references Alexion Pharmaceuticals Inc.’s rare blood disease medicine, Soliris.
Codenamed SB12 (Eculizumab), Samsung’s Epysqli has shown the same clinical effects as Alexion’s blockbuster treatment Soliris in paroxysmal nocturnal hemoglobinuria (PNH) patients.
Alexion, the developer of Soliris, was taken over by AstraZeneca plc, a British-Swedish multinational pharmaceutical and biotechnology company, in 2021.
Soliris, the original Eculizumab drug, obtained US Food and Drug Administration approval in 2007 and has since posted billions of dollars in sales globally.
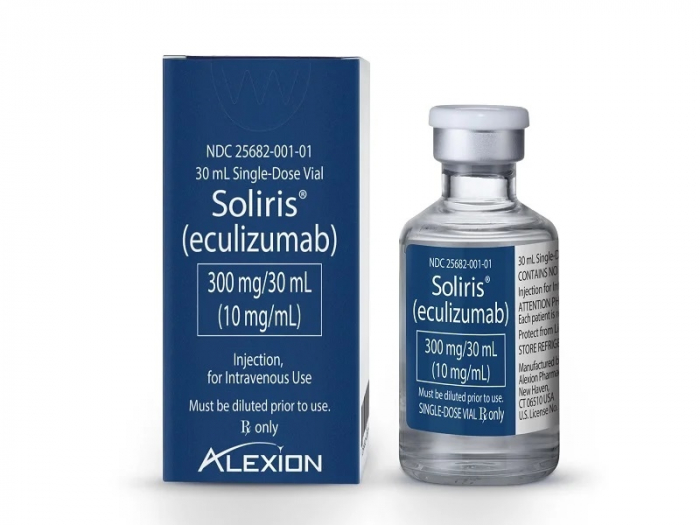
Samsung in May obtained regulatory approval from the European Commission to sell Epysqli in the region.
LATECOMER SET TO GROW BIG
During the European Hematology Association (EHA)’s annual meeting in Frankfurt, Germany June 8-11, Peffault de Latour, a hematology professor in the Hematology and Bone Marrow Transplant Department of the Saint-Louis Hospital in Paris, praised Samsung’s Epysqli.
“Samsung Bioepis is a latecomer to the biopharmaceutical industry. But the company is expected to lead the industry in a relatively short span of time,” he said.
Samsung said it has set up a promotional booth at the EHA meeting for the first time since the company’s founding in 2012.

The Korean company is unveiling Epysqli after eight years of development and clinical trials to offer PNH patients a reasonable alternative to Soliris, which costs between 400 million and 500 million won per patient a year.
PNH occurs at a rate of 15 cases per million people, so the number of patients is relatively small, making it challenging for biosimilar firms to recruit patients for clinical trials and venture into the business.
Samsung said it aims to penetrate the niche market to compete with the likes of Soliris, Ultomiris, AstraZeneca's another PNH medicine, and Amgen’s Eculizumab biosimilar Bekemv.
“Epysqli is in a better position than its rivals. It contains no sorbitol, an ingredient known to cause allergic reactions. It also has clinical data on pregnant women and boasts rapid efficacy,” said Jang Jun-ho, a professor at the Department of Hematology and Oncology of Samsung Medical Center in Seoul who was in charge of the biosimilar's phase 3 clinical trials.
TEN BIOSIMILARS IN THE PIPELINE
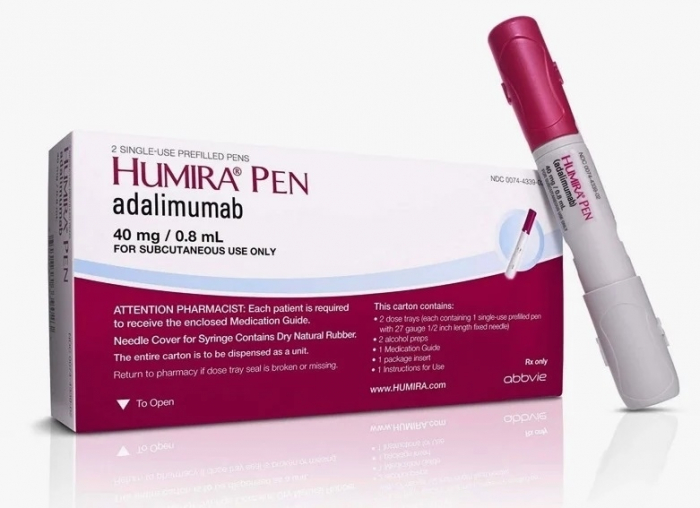
With 10 biosimilar products in the pipeline, Samsung Bioepis plans to target the US and European markets in the second half of this year with its products Epysqli and Hadlima, a biosimilar referencing AbbVie’s blockbuster drug Humira, a treatment for rheumatoid arthritis, Crohn's disease and ulcerative colitis.
The global markets for Humira and Soliris are estimated at 27 trillion won and 5 trillion won each annually.
Industry watchers said about 10 Humira biosimilars will hit the 20 trillion won US market by the end of this year.
Samsung’s Hadlima has completed interchangeability clinical tests with Humira, meaning the medicine can be prescribed at a pharmacy without a doctor’s prior consent.
“We will make Hadlima the top Humira biosimilar in the US market by 2025,” said Park Sang-jin, vice president and head of Samsung Bioepis’ commercial division.
Write to Dae-Kyu Ahn at powerzanic@hankyung.com
In-Soo Nam edited this article.
More to Read
-
 Bio & PharmaSamsung Bioepis’ SB12 shows same clinical effects as Alexion’s Soliris
Bio & PharmaSamsung Bioepis’ SB12 shows same clinical effects as Alexion’s SolirisJun 14, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaSamsung Bioepis gets approval to sell biosimilar in Europe
Bio & PharmaSamsung Bioepis gets approval to sell biosimilar in EuropeMay 31, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSamsung Bioepis sees greater market for Byooviz eye disease biosimilar
Bio & PharmaSamsung Bioepis sees greater market for Byooviz eye disease biosimilarJul 18, 2022 (Gmt+09:00)
2 Min read -
 PharmaceuticalsSamsung Bioepis to double biosimilars, may resume Nasdaq listing plans
PharmaceuticalsSamsung Bioepis to double biosimilars, may resume Nasdaq listing plansFeb 24, 2022 (Gmt+09:00)
3 Min read -
 PharmaceuticalsSamsung Bioepis posts $1.26 billion in global biosimilar sales
PharmaceuticalsSamsung Bioepis posts $1.26 billion in global biosimilar salesFeb 18, 2022 (Gmt+09:00)
2 Min read
Comment 0
LOG IN


