Bio & Pharma
Celltrion gets sales approval for Yuflyma in Japan
The company will sell the biosimilar of Humira in 47 countries, including Japan, the US, and Europe
By Sep 26, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Mirae Asset to be named Korea Post’s core real estate fund operator


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


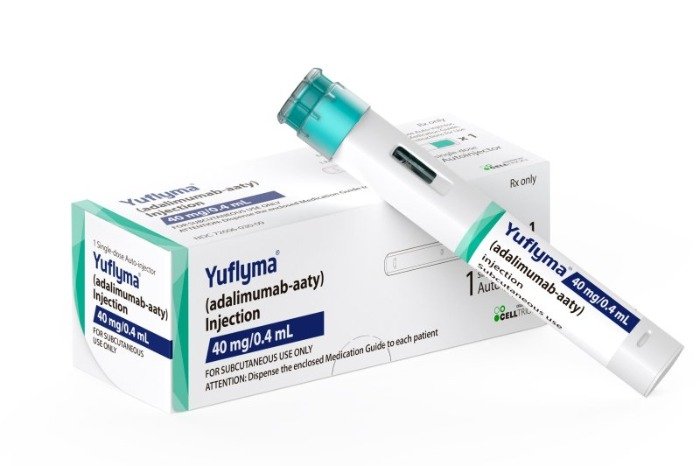
South Korea's biopharmaceutical company Celltrion Inc. announced on Tuesday that it has secured approval from the Japanese Ministry of Health, Labour, and Welfare to market Yuflyma, a biosimilar of the acclaimed autoimmune disease drug, Humira.
This approval enables Yuflyma to be used for the principal applications in Japan that Humira addresses, including rheumatoid arthritis, inflammatory bowel disease, and psoriasis.
Subsequent to approvals in the US and Europe, Yuflyma has now gained approval in a total of 47 countries.
Humira, developed by the multinational pharmaceutical company AbbVie, features the active ingredient adalimumab and is distinguished as a blockbuster medicine, recording sales of $21.2 billion as of last year.
In alignment with its ongoing strategies, Celltrion is set to expedite the integration of Yuflyma into the Japanese market.
The company intends to leverage the experiences and insights gained from the successful launch and establishment of other drugs in Japan, such as Herzuma for breast and stomach cancer, Vegzelma for metastatic colorectal cancer, and Remsima for autoimmune diseases.
Write to Jeong Min Nam at peux@hankyung.com
More to Read
-
 Bio & PharmaCelltrion Healthcare to supply Yuflyma to five regional gov’ts in Italy
Bio & PharmaCelltrion Healthcare to supply Yuflyma to five regional gov’ts in ItalySep 18, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion Healthcare launches Humira biosimilar Yuflyma in US
Bio & PharmaCelltrion Healthcare launches Humira biosimilar Yuflyma in USJul 04, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion applies for EMA approval of smaller dose formulation of Yuflyma
Bio & PharmaCelltrion applies for EMA approval of smaller dose formulation of YuflymaJan 16, 2023 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


