Bio & Pharma
Lunit receives $1 mn in tech fees from Guardant Health of US
The AI-based pathology analysis solution Lunit Scope PD-L1 has received crucial verification from CLIA
By Mar 08, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


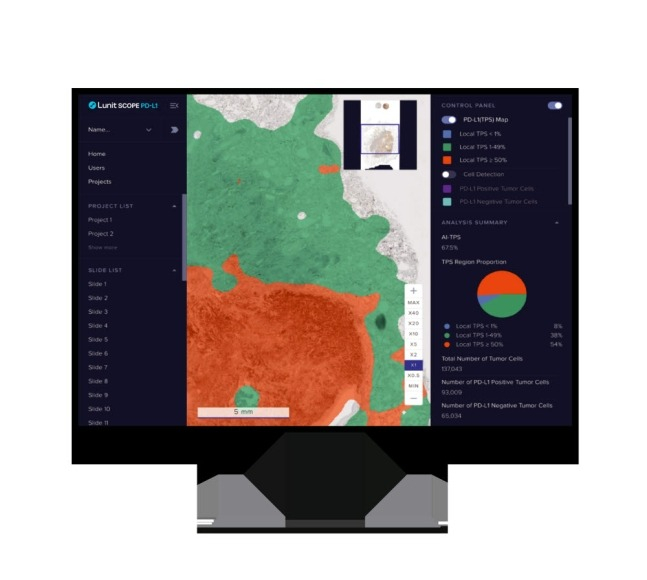
The US operations of South Korea's artificial intelligence (AI) medical company Lunit have received a major boost after the company got the green light for use of its products at facilities requiring Clinical Laboratory Improvement Amendments (CLIA) certification, an American lab standard.
Lunit on Tuesday said it will receive $1 million in technology fees from Guardant Health, a leading American company in liquid biopsy that tests cancer markers with blood that also supervises US distribution of Lunit Scope PD-L1, an AI-based pathology analysis solution. Guardant Health's products are used by 80% of US-based professors in hemato-oncology.
Guardant Health's verification procedures led to certification of Lunit Scope PD-L1 for use at CLIA-certified labs, with the product expected to see boosted sales on the American market thanks to the certification. The technology fee earned stemmed from successful verification of laboratory developed tests (LDTs) used at CLIA-certified facilities.
Lunit Scope PD-L1 is grafted on Guardant Health's tissue biopsy test Guardant360 TissueNext to accurately analyze the degree of expression of the cancer biomarker PD-L1. This certification will allow Lunit to expand this product's use to clinical tests such as those for non-small cell lung cancer, triple-negative breast cancer and bladder cancer.
In the first half of this year, the company will complete additional LDT verification for 10 cancer types.
Write to Ji-Hyun Lee at bluesky@hankyung.com
More to Read
-
 Artificial intelligenceAI medical software company Lunit to open subsidiary in Europe
Artificial intelligenceAI medical software company Lunit to open subsidiary in EuropeFeb 17, 2023 (Gmt+09:00)
1 Min read -
 Artificial intelligenceS.Korea's Lunit supplies breast cancer imaging solution to UAE
Artificial intelligenceS.Korea's Lunit supplies breast cancer imaging solution to UAEFeb 09, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaLunit launches AI-based pathology analysis solution with US firm
Bio & PharmaLunit launches AI-based pathology analysis solution with US firmFeb 01, 2023 (Gmt+09:00)
1 Min read -
 Artificial intelligenceLunit to export breast cancer detecting solutions to Hong Kong, Mongolia
Artificial intelligenceLunit to export breast cancer detecting solutions to Hong Kong, MongoliaDec 22, 2022 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


