Vaccine business
SK Bioscience eyes $900 mn annual sales from vaccine business
By Feb 23, 2021 (Gmt+09:00)
2
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator



South Korea’s SK Bioscience Co. is looking to post 1 trillion won ($900 million) in annual revenue from its vaccine business as the biopharmaceutical unit of SK Group expects a steady stream of contract manufacturing organization (CMO) orders from global firms.
During an online press conference on Feb. 23, Chief Executive Ahn Jae-yong said the company also plans to unveil two versions of its own COVID-19 vaccine in the first half of next year, which it expects to help end the pandemic.
“We’re receiving CMO requests from global pharma companies even when our nine vaccine production lines at the Andong plant are running at full capacity,” said CEO Ahn.
The media briefing comes ahead of SK’s planned initial public share sale in March.
A spin-off of SK Chemicals Co.'s vaccine division, SK Bioscience is set to list on the main local bourse on Mar. 18 to raise up to $1.3 billion, in what is expected to be one of the country’s blockbuster IPOs this year.
The SK Group unit will price its shares in a band of 49,000 and 65,000 won ($44 and $58.3) apiece, valuing its entire company at up to 5 trillion won. In an initial public offering, it will float 22.95 million shares, including 15.3 million new issues.
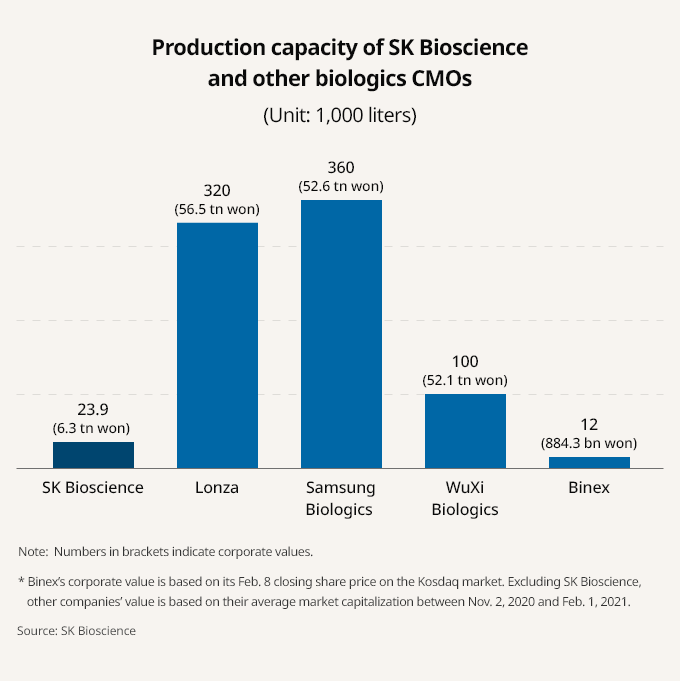
ADDITIONAL CMO ORDERS FLOOD IN
At Tuesday’s press conference, the CEO said the company expects 300 billion won in annual revenue from its CMO business alone, and if the development and sale of its own vaccine products are added, its revenue could eventually rise to 1 trillion won.

SK has been producing AstraZeneca’s COVID-19 vaccine since September 2020. It also inked a contract development and manufacturing organization (CDMO) order for a coronavirus vaccine candidate developed by Novavax last year.
The Korean vaccine maker said it may be getting manufacturing orders from France’s Sanofi S.A. and British multinational pharmaceutical company GlaxoSmithKline plc (GSK).
IPO PROCEEDS TO GO TO FACILITY EXPANSION
SK Bioscience said it is raising money via the IPO to expand its research and production facilities; develop new pipelines; foster partnerships; and enhance its vaccine portfolio.
CEO Ahn said on Tuesday the company will spend 400 billion won in facility investment, 100 billion won on securing platform technology and up to 200 billion won on R&D projects.
The company will also invest about 100 billion won on expanding its overseas manufacturing facilities, he said.
NH Investment & Securities is the IPO lead manager.
Write to Ju-hyun Lee and Woo-Sub Kim at deep@hankyung.com
In-Soo Nam edited this article.
More to Read
-
 Business & PoliticsTrump Jr. meets Korean business chiefs in back-to-back sessions
Business & PoliticsTrump Jr. meets Korean business chiefs in back-to-back sessionsApr 30, 2025 (Gmt+09:00)
-
 Korean chipmakersSamsung in talks to supply customized HBM4 to Nvidia, Broadcom, Google
Korean chipmakersSamsung in talks to supply customized HBM4 to Nvidia, Broadcom, GoogleApr 30, 2025 (Gmt+09:00)
-
 EnergyLS Cable breaks ground on $681 mn underwater cable plant in Chesapeake
EnergyLS Cable breaks ground on $681 mn underwater cable plant in ChesapeakeApr 29, 2025 (Gmt+09:00)
-
 Business & PoliticsUS tariffs add risk premium to dollar assets: Maurice Obstfeld
Business & PoliticsUS tariffs add risk premium to dollar assets: Maurice ObstfeldApr 29, 2025 (Gmt+09:00)
-

Comment 0
LOG IN


