COVID-19 treatment
Celltrion to export COVID-19 treatment to Europe in 2021
To ship Regkirona to nine European countries by year-end; in talks with 47 nations across the globe
By Nov 30, 2021 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Mirae Asset to be named Korea Post’s core real estate fund operator


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


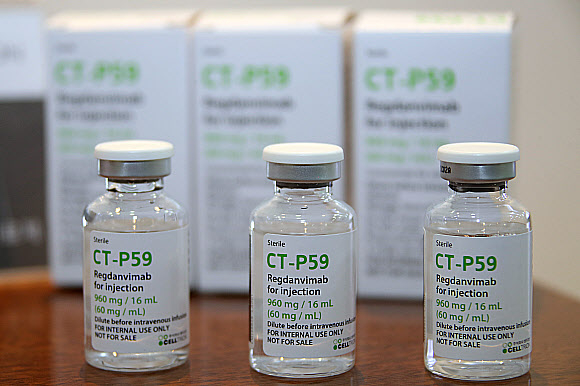
South Korea’s pharmaceutical giant Celltrion Inc. is set to export its COVID-19 treatment Regkirona to Europe by the end of this year as it seeks a larger market share worldwide amid a resurgence in the pandemic with the onset of the Omicron variant.
Celltrion Healthcare Co., which handles Regkirona’s global sales, said on Nov. 30 that it will deliver 150,000 vials of the treatment, enough for 50,000 patients, to nine countries in Europe by year-end. It did not identify the nine countries.
Celltrion Healthcare is also in talks with 47 nations in Europe, Asia, South America and the Middle East for exports, according to the company.
“More countries are inquiring about the supply of Regkirona, so we expect exports to increase,” said a company official.
COVID-19 cases are again surgin around the globe, especially in Europe. The number of new confirmed cases in the region rose 11% in one week to 2.4 million, representing 67% of the total patients in the world as of Nov. 21.
Regkirona was the first monoclonal antibody drug, along with Ronapreve, co-developed by Regeneron Pharmaceuticals Inc. and Roche Registration GmbH, for the pandemic approved by the European Commission.
Regkirona drew strong demand as the drug’s high efficacy and safety were proven through tests of more than 20,000 patients in South Korea, local industry sources said.
“It is getting more reliable thanks to growing prescription data to prove its effective treatment and safety,” said the company official.
Celltrion aims to sell Regkirona in the US. The company is in discussions with the US Food and Drug Administration (FDA) for emergency use authorization.
Write to Jae-young Han at jyhan@hankyung.com
Jongwoo Cheon edited this article.
More to Read
-
 COVID-19 treatmentCelltrion wins COVID-19 treatment approval from Europe
COVID-19 treatmentCelltrion wins COVID-19 treatment approval from EuropeNov 15, 2021 (Gmt+09:00)
4 Min read -
 Covid-19 treatmentBrazil gives emergency use nod for Celltrion’s COVID-19 treatment
Covid-19 treatmentBrazil gives emergency use nod for Celltrion’s COVID-19 treatmentAug 13, 2021 (Gmt+09:00)
1 Min read -
 Covid-19 treatmentIndonesia grants emergency use approval for Celltrion’s COVID-19 treatment
Covid-19 treatmentIndonesia grants emergency use approval for Celltrion’s COVID-19 treatmentJul 20, 2021 (Gmt+09:00)
1 Min read -
 COVID-19 treatmentCelltrion’s COVID-19 treatment reduces incidence of severity by 70%
COVID-19 treatmentCelltrion’s COVID-19 treatment reduces incidence of severity by 70%Jul 13, 2021 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


