SK Bioscience to develop 2nd-generation Ebola vaccine
The S.Korean pharmaceutical firm signs a joint development agreement with Hilleman Labs for a new Zaire Ebola virus vaccine
By Nov 22, 2023 (Gmt+09:00)
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


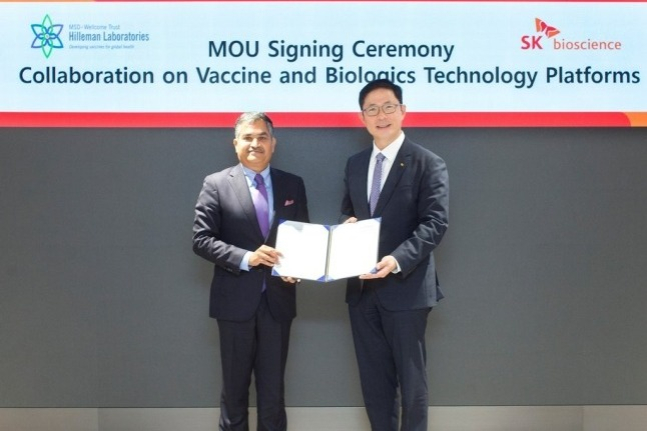
South Korea's SK Bioscience Co. announced on Wednesday that it signed a business agreement with the international non-profit research organization Hilleman Laboratories for the joint development of a second-generation Ebola Zaire virus vaccine.
Under the agreement, SK Bioscience and Hilleman Laboratories plan to develop a product that improves upon the existing Ebola vaccine "Ervebo" from the global pharmaceutical company MSD in terms of vaccine production processes, production efficiency, and heat stability.
Hilleman Laboratories is a research organization established in 2009 through a joint investment by pharmaceutical company MSD and the British charitable foundation for medical research Wellcome Trust.
Once the vaccine is developed, SK Bioscience and Hilleman Laboratories aim to ensure the competitive pricing of the vaccine and supply it to low- and middle-income countries.
Both parties had previously entered into an agreement in October last year for joint research and development of new vaccines and platforms. This new agreement specifies the vaccine to develop and delineates the roles for vaccine development, according to SK Bioscience.
In May this year, the company also signed a deal with MSD for the outsourced production of the candidate material for the next-generation Ebola Zaire vaccine.
Upon the commercialization of the developed vaccine, SK Bio plans to outsource the production of the second-generation Ebola vaccine, supplying it globally from its own vaccine facility Andong L House.
"Developing a vaccine to prevent viruses that cause highly fatal diseases such as Ebola is an essential task to safeguard the survival of humanity," CEO of SK Bioscience Ahn Jae-yong said.
He further expressed the company's commitment to successfully completing the project, contributing to overcoming diseases and expanding collaboration with overseas companies and institutions to grow into a global enterprise.
Write to Ji-Hyun Lee at bluesky@hankyung.com
-
 Bio & PharmaSK Bioscience resumes output of flu vaccine after two years
Bio & PharmaSK Bioscience resumes output of flu vaccine after two yearsAug 24, 2023 (Gmt+09:00)
2 Min read -
 Bio & PharmaSK Bioscience to export $51 bn flu vaccine to Thailand
Bio & PharmaSK Bioscience to export $51 bn flu vaccine to ThailandAug 21, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Bioscience buys 6.5 million Novavax shares for $83.4 million
Bio & PharmaSK Bioscience buys 6.5 million Novavax shares for $83.4 millionAug 09, 2023 (Gmt+09:00)
2 Min read -
 Bio & PharmaSK Bioscience, Thai’s GPO to jointly boost vaccine infrastructure
Bio & PharmaSK Bioscience, Thai’s GPO to jointly boost vaccine infrastructureJul 05, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaS.Korea's SK Bioscience to resume domestic flu vaccine output
Bio & PharmaS.Korea's SK Bioscience to resume domestic flu vaccine outputJun 14, 2023 (Gmt+09:00)
1 Min read


