SK Bioscience resumes output of flu vaccine after two years
The S.Korean market will get five million doses of SKYCellflu by early 2024, with 10 countries approving its use
By Aug 24, 2023 (Gmt+09:00)
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


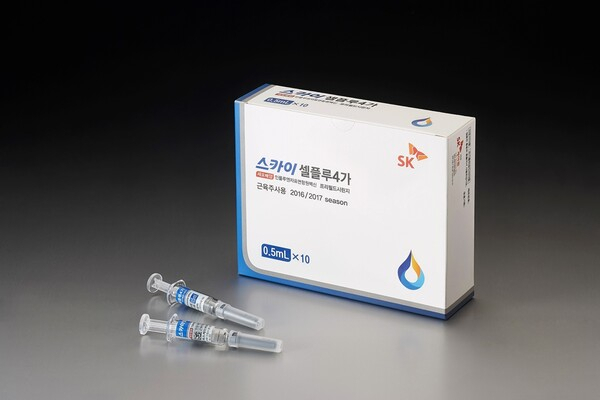
SK Bioscience Co., the drug-making unit of South Korea’s SK Group, on Wednesday shipped SKYCellflu, its vaccine for the common flu, for the first time in two years.
By early next year, the domestic market will get about five million doses of SKYCellflu, the world's first cell culture-based flu vaccine effective against four virus types.
SK Bioscience in 2020 produced 9.1 million doses of SKYCellflu for sales of 63.8 billion won ($48 million), making the vaccine the country's most used. Production was halted in 2021 and last year, however, so that the company could focus on COVID-19 vaccines.
This year, select facilities are making the coronavirus vaccine of Novavax, an American vaccine maker in which SK Bioscience this month bought a 7% stake.
"Around two months are needed just to clean and disinfect the plant to change production," said Lee Sang-kyun, chief of L House, SK Bioscience's plant in Andong, North Gyeongsang Province. "Production resumed after two years with no quality issues."
Of the five million doses for domestic supply, 2.42 million will be for national vaccination, the most among the six companies taking part in the government's flu vaccine procurement including GC Biopharma and Sanofi-Aventis Korea. The remaining 2.58 million doses will be sold on the private market, and administering of SKYCellflu will begin late this month at hospitals and clinics.
The vaccine has received approval in 10 countries including Malaysia, Thailand, Singapore and Mongolia. "We will expand our global competitiveness by getting additional approval in about 10 countries," Lee said.
A cell culture vaccine is made by culturing stem cells from animals, while most vaccine makers use the fertilized chicken egg method.
"The fertilized chicken egg method excludes patients with allergies to eggs, and another problem is frequent occurrence of mutations in the process of amplifying viruses in eggs," an SK Bioscience source said.
The cell culture method has no virus mutations, however, with a preventive effect 11% higher than the other method.
"There is little risk of vaccine hypersensitivity because of no preservatives or antibiotics," the source said. "Our vaccine's advantages are a short production period and possibility of mass output for easy supply during a flu outbreak."
Write to Hyun-Ah Oh at 5hyun@hankyung.com
-
 Bio & PharmaSK Bioscience to export $51 bn flu vaccine to Thailand
Bio & PharmaSK Bioscience to export $51 bn flu vaccine to ThailandAug 21, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Bioscience buys 6.5 million Novavax shares for $83.4 million
Bio & PharmaSK Bioscience buys 6.5 million Novavax shares for $83.4 millionAug 09, 2023 (Gmt+09:00)
2 Min read -
 Bio & PharmaSK Bioscience, Thai’s GPO to jointly boost vaccine infrastructure
Bio & PharmaSK Bioscience, Thai’s GPO to jointly boost vaccine infrastructureJul 05, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaS.Korea's SK Bioscience to resume domestic flu vaccine output
Bio & PharmaS.Korea's SK Bioscience to resume domestic flu vaccine outputJun 14, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Bioscience’s COVID-19 vaccine gets approval in UK
Bio & PharmaSK Bioscience’s COVID-19 vaccine gets approval in UKMay 30, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Bioscience to produce MSD’s new Ebola vaccine candidate
Bio & PharmaSK Bioscience to produce MSD’s new Ebola vaccine candidateMay 08, 2023 (Gmt+09:00)
1 Min read


