Bio & Pharma
Celltrion to supply Remsima to Brazil gov’t in three-year row
Celltrion Healthcare anticipates over 80% local market share, synergy with Remsima SC in selling
By Nov 10, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


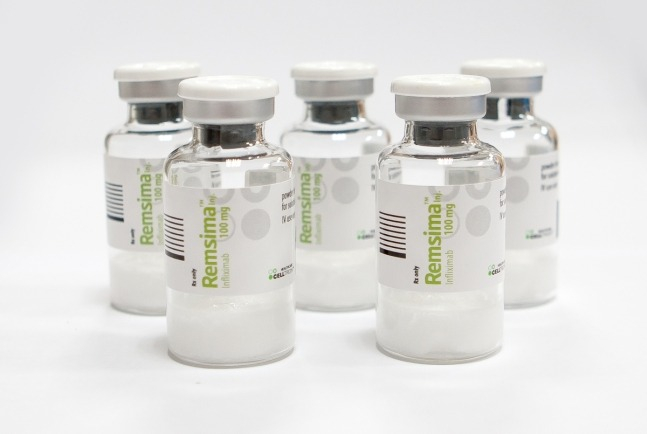
South Korea's Celltrion Healthcare Co. announced on Friday its autoimmune disease treatment Remsima (ingredient: infliximab) has won a bid for the Brazilian federal government for three consecutive years.
The company is set to supply 360,000 vials of Remsima in the first half of next year. The federal government's demand constitutes 60% of the Brazilian infliximab component pharmaceutical market, and Celltrion Healthcare has been the sole supplier of Remsima to the federal government for the past two years.
The company also noted its consistent success in securing public procurement deals through bids put up by state governments.
Celltrion Healthcare expects that the recently launched Remsima SC, the Remsima subcutaneous injection version, introduced in Brazil in July, will generate synergies in sales in the future.
Celltrion Healthcare has also introduced Remsima SC in other Central and South American countries such as Peru, Colombia, and Chile. The company is gearing up for the release of additional products, including a biosimilar for autoimmune diseases Yuflyma, and a biosimilar used in metastatic colorectal cancer Vegzelma starting next year.
Write to Dae-Kyu Ahn at powerzanic@hankyung.com
More to Read
-
 Bio & PharmaCelltrion Healthcare wins Remsima order in France, Italy
Bio & PharmaCelltrion Healthcare wins Remsima order in France, ItalyOct 18, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion Healthcare to supply Yuflyma to US pharmacy chain
Bio & PharmaCelltrion Healthcare to supply Yuflyma to US pharmacy chainOct 16, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion Healthcare to supply Yuflyma to five regional gov’ts in Italy
Bio & PharmaCelltrion Healthcare to supply Yuflyma to five regional gov’ts in ItalySep 18, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion Healthcare launches Humira biosimilar Yuflyma in US
Bio & PharmaCelltrion Healthcare launches Humira biosimilar Yuflyma in USJul 04, 2023 (Gmt+09:00)
1 Min read -
Comment 0
LOG IN


