Bio & Pharma
Daewoong Pharma's GER drug gets product OK from Mexico
With Latin America's top medicine market, the country is the fourth to grant approval after the Philippines, Ecuador and Chile
By Oct 19, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


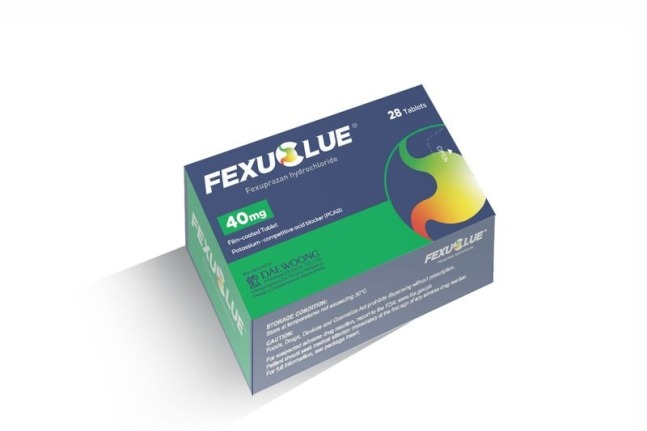
South Korea’s Daewoong Pharmaceutical on Wednesday said its gastroesophageal reflux (GER) disease drug Fexuclue (active ingredient fexuprazan hydrochloride) has received product approval from Mexico’s COFEPRIS, or the Federal Committee for Protection from Sanitary Risks.
Mexico is the fourth country excluding South Korea to authorize Fexuclue, which was released in its nation of origin two years ago.
Mexico has the largest pharmaceutical market in Central and South America, with its ulcer drug sector alone worth $205 million (270 billion won) last year according to the US-based pharmaceutical market observer IQVIA.
As a new drug for GER disease in the potassium-competitive acid blocker (P-CAB) class, Fexuclue improves the shortcomings of other proton pump inhibitors like time needed to take effect, acid secretion at night and dietary influences.
By 2025, Daewoong Pharmaceutical seeks to secure approval for Fexaclue from 30 countries. So far, it has received the OK from four of the 12 countries it has applied with and plans to raise that number to a cumulative 20 within the year.
"The news of our product approval from Mexico, the No. 1 market in Central and South America, following those received early this year from Ecuador and Chile, shows how Fexuclue’s value continues to gain recognition," Daewoong Pharmaceutical CEO Jeon Seng-ho said.
Write to In-Hyuk Park at hyuk@hankyung.com
More to Read
-
 Bio & PharmaDaewoong to develop eye drops for diabetic retinopathy
Bio & PharmaDaewoong to develop eye drops for diabetic retinopathySep 15, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaDaewoong gets patent of Nabota as migraine drug in US
Bio & PharmaDaewoong gets patent of Nabota as migraine drug in USSep 07, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaDaewoong applies for approval of Envlo in Saudi Arabia
Bio & PharmaDaewoong applies for approval of Envlo in Saudi ArabiaAug 25, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaDaewoong launches GERD treatment Fexuclu in Philippines
Bio & PharmaDaewoong launches GERD treatment Fexuclu in PhilippinesAug 01, 2023 (Gmt+09:00)
1 Min read -
Comment 0
LOG IN


