Bio & Pharma
Daewoong gets patent of Nabota as migraine drug in US
The treatment's exclusive rights protected until 2041, S.Korean pharmaceutical company plants to expand indications
By Sep 07, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


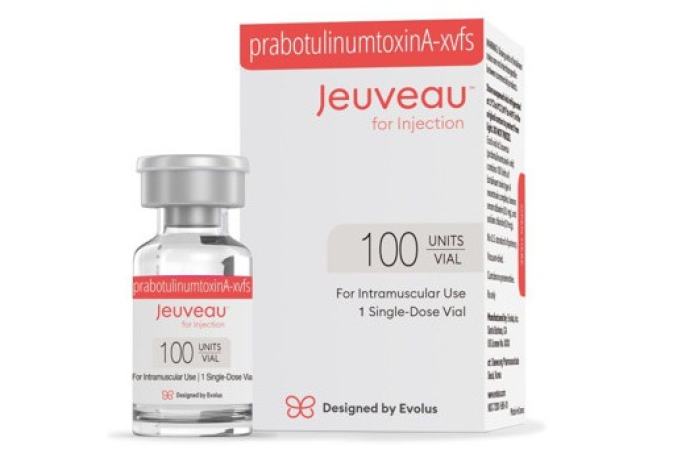
South Korea's Daewoong Pharmaceutical Co. announced on Thursday that it has received a patent from the US Patent and Trademark Office for the use of its botulinum toxin Nabota (American name: Jeuveau) in the treatment of migraines through its US partner AEON Biopharma.
This patent provides exclusive rights in the US until 2041. The company explained that the patent was granted based on factors such as a reduced number of administrations compared to existing botulinum toxin products and improvements in convenience through changes in administration sites.
With this patent, Daewoong expects that the phase 2 clinical trials of Nabota for episodic and chronic migraines currently being conducted by AEON Biopharma in the US will progress smoothly.
Botulinum toxin, commonly referred to as botox, is a biopharmaceutical used in cosmetic procedures such as improving glabellar lines or square jaw correction. Half of its worldwide sales come from the therapeutic market and the other half from the cosmetic market.
Daewoong Pharmaceutical plans to expand the indications for Nabota to include episodic and chronic migraines, cervical dystonia, gastroparesis and post-traumatic stress disorder (PTSD).
The major results of phase 2 clinical trials for episodic migraines are expected to be announced in the second half of this year, while major results for chronic migraines are expected next year.
Phase 2 clinical trials for cervical dystonia are planned to conclude by the end of this year, with plans to enter phase 3 next year. Clinical phase 2 plans for gastroparesis are currently in progress. Preclinical work is underway for PTSD.
"With the acquisition of this patent, we expect to become the world's second company to receive approval for the treatment of migraines," said Park Seongsoo, Vice President of Daewoong Pharmaceutical. "Through close cooperation with AEON Biopharma, we will achieve the rapid entry of Nabota into the therapeutic market."
Write to Ye-Na Kim at yena@hankyung.com
More to Read
-
 Bio & PharmaDaewoong launches GERD treatment Fexuclu in Philippines
Bio & PharmaDaewoong launches GERD treatment Fexuclu in PhilippinesAug 01, 2023 (Gmt+09:00)
1 Min read -
-
 Bio & PharmaDaewoong Pharmaceutical’s cumulative tech exports exceed $870 mn
Bio & PharmaDaewoong Pharmaceutical’s cumulative tech exports exceed $870 mnMay 12, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaDaewoong Pharma to build third botulinum toxin plant for $746 mn
Bio & PharmaDaewoong Pharma to build third botulinum toxin plant for $746 mnMay 03, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaDaewoong Pharma, UK firm to jointly develop autoimmune disease drug
Bio & PharmaDaewoong Pharma, UK firm to jointly develop autoimmune disease drugApr 18, 2023 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


