Bio & Pharma
SK Biopharm’s Cenobamate gets nod in Canada, to boost US sales
With global sales of the antiepileptic treatment, SK hopes to become one of the world's top 10 healthcare firms by 2030
By Jun 15, 2023 (Gmt+09:00)
2
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator



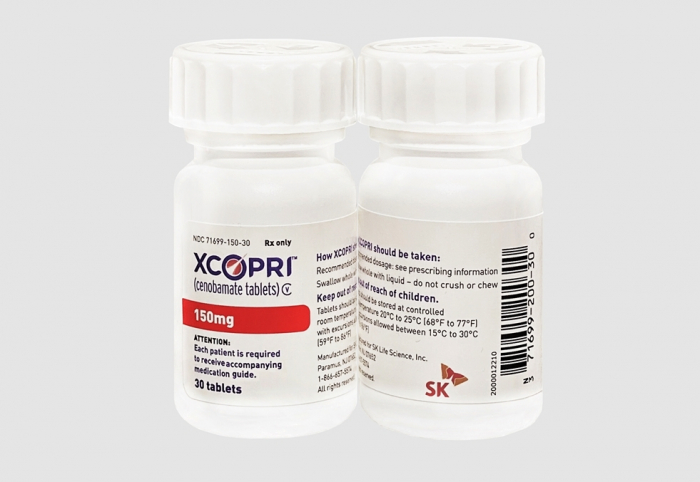
South Korea’s SK Biopharmaceuticals Co. has obtained the sales approval for its antiepileptic treatment Cenobamate in Canada, paving the way for its business expansion throughout North America, including the US.
The biotech unit of Korea’s energy-to-battery conglomerate SK Group said on Thursday it received a notice of compliance from Health Canada for the sale of Cenobamate in the country under the brand name Xcopri.
The sales and marketing of the medication used to treat partial onset epileptic seizures will be carried out through SK’s Canadian partner, Paladin Labs Inc., a Montreal-based pharmaceutical firm.
Cenobamate is the first epilepsy treatment drug that a South Korean company has developed on its own from candidate substance discovery to clinical trials, licensing and sales.
In the US, SK obtained approval from the Food and Drug Administration (FDA) in 2019 and began selling Xcopri the following year through its own sales channels.
CANADA, ONE OF TOP 10 MARKETS
SK said its sales in Canada, one of the world’s top 10 pharmaceutical markets, would boost its presence in North America, which accounts for more than half the $6.1 billion global epilepsy treatment market as of 2019. The size of the US market stood at $3.3 billion while the Canadian market was $220 million at the end of 2019.
Epilepsy is one of the most common neurological diseases, affecting millions of people around the world.
Last July, SK signed a deal to license its Cenobamate manufacturing technology to Brazil’s Eurofarma Laboratórios S.A. to enter the Latin American market.
With the technology transfer, SK said at the time that it expects to sell Cenobamate in 17 Latin American countries via its Brazilian partner.
In December 2021, it took the drug to the UK following earlier launches in Germany, Denmark and Sweden.

In Europe, the company has so far launched the medicine under the brand name Ontozry in 18 countries, including Italy and France. It plans to obtain sales approval in five additional countries by the end of this year.
In Asia, Cenobamate is in phase 3 clinical trials.
XCOPRI TO BOOST SK’S EARNINGS
The company earned 53.9 billion won ($42 million) from the sale of Cenobamate in the first quarter, nearly double its revenue of the medicine in the year-earlier period.

SK Inc., the investment and holding company of SK Group, said earlier this year that it expects significant growth in its biopharmaceutical business from 2024 when its investment in bio-related startups and venture companies are set to produce tangible results.
SK Biopharmaceuticals aims to become one of the world’s top 10 healthcare companies by 2030 with an enterprise value of 50 trillion won by then.
Company executives said they will redouble marketing efforts globally and particularly in the US to raise its corporate value to 25 trillion won by 2025.
Write to Dae-Kyu Ahn at powerzanic@hankyung.com
In-Soo Nam edited this article.
More to Read
-
 Bio & PharmaSK Biopharmaceuticals gets marketing approval for epilepsy drug in Israel
Bio & PharmaSK Biopharmaceuticals gets marketing approval for epilepsy drug in IsraelMay 03, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharma's epilepsy treatment posts impressive growth in US
Bio & PharmaSK Biopharma's epilepsy treatment posts impressive growth in USNov 11, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharmaceuticals signs epilepsy drug deal with Brazil’s Eurofarma
Bio & PharmaSK Biopharmaceuticals signs epilepsy drug deal with Brazil’s EurofarmaJul 15, 2022 (Gmt+09:00)
2 Min read -
 PharmaceuticalsSK Biopharm aims to make it to global top 10 healthcare firms by 2030
PharmaceuticalsSK Biopharm aims to make it to global top 10 healthcare firms by 2030Jul 02, 2021 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


