Bio & Pharma
SK Inc. eyes significant growth in bio business from 2024
SK Biopharma aims to become one of the top 10 healthcare firms by 2030 with $44 billion in corporate value
By Jan 13, 2023 (Gmt+09:00)
2
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator



SAN FRANCISCO – SK Inc., the investment and holding company of South Korea’s SK Group, expects significant growth in its biopharmaceutical business from 2024 when its investment in bio-related startups and venture companies are poised to produce tangible results.
At the SK Bio Night, a networking event held in San Francisco on Wednesday, SK executives unveiled the group’s vision for global expansion to its partners.
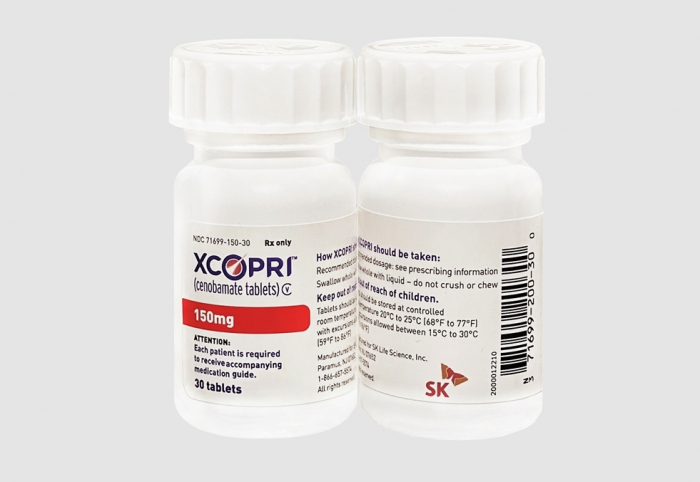
SK Biopharmaceuticals Co. Chief Executive Lee Donghoon said the company’s business focus will be on increasing sales of its epilepsy treatment Cenobamate, expanding its life science product portfolio and strengthening its global partnerships.
“Through our direct sales strategy, we have successfully soft-landed Cenobamate in the US market,” he said. “Now, we’re ready to take off globally.”
In May 2020, SK began selling Cenobamate, a medication to treat partial-onset epileptic seizures, in the US under the brand name Xcopri.
The company has also launched the product in five European countries, including France, Germany and Italy, and Latin American countries, including Brazil and Mexico.

Last July, SK signed a deal to license its Cenobamate technology to Brazil’s Eurofarma Laboratórios S.A.
With the technology transfer, SK aims to sell Cenobamate in 17 Latin American countries via its Brazilian partner.
In December 2021, it took the drug to the UK following earlier launches in Germany, Denmark and Sweden for the European market.
In Asia, Cenobamate is in phase 3 clinical trials.
ADC BUSINESS USING CMO INFRASTRUCTURE
At the SK Bio Night, SK Bio Investment Center chief Kim Yeontae said the company is also looking closely at antibody-drug conjugates (ADCs), a next-generation technology designed to specifically target cancer cells.
“We believe ADC is an area where SK can best show its technological prowess,” he said. “We plan to use our contract manufacturing organization, or CMO, facilities for the ADC business.”
Korea’s two other bioscience giants – Samsung Biologics Co. and Lotte Biologics Co. – have already said they were entering the ADC market.
In the CMO business, SK has invested in a number of bio startups, which are expected to produce tangible results from 2024, Kim said.
SK Pharmteco Chief Executive Joerg Ahlgrimm said the company aims to achieve $2 billion in annual sales by 2025 and expand its CMO production capacity to 75,000 square meters through a facility ramp-up by 2026.
SK Inc. established SK Pharmteco in 2019 to integrate the CDMO businesses in Korea, the US and Europe. SK Biopharmaceuticals focuses on the research and development of new drugs.
SK Biopharmaceuticals CEO Lee said he expects the company, which is losing money, to turn to profit by the end of this year.
The company earlier said it aims to become one of the world’s top 10 healthcare companies by 2030 with an enterprise value of 50 trillion won ($44 billion) by then.
Write to Jeong-Min Nam at peux@hankyung.com
In-Soo Nam edited this article.
More to Read
-
 Bio & PharmaSK bio families to present visions to global investors at SK Bio Night
Bio & PharmaSK bio families to present visions to global investors at SK Bio NightJan 10, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharmaceuticals launches epilepsy drug in France
Bio & PharmaSK Biopharmaceuticals launches epilepsy drug in FranceDec 09, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharmaceuticals signs epilepsy drug deal with Brazil’s Eurofarma
Bio & PharmaSK Biopharmaceuticals signs epilepsy drug deal with Brazil’s EurofarmaJul 15, 2022 (Gmt+09:00)
2 Min read -
 PharmaceuticalsSK Biopharm aims to make it to global top 10 healthcare firms by 2030
PharmaceuticalsSK Biopharm aims to make it to global top 10 healthcare firms by 2030Jul 02, 2021 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


