Bio & Pharma
Hanmi's Rolvedon set to post $10 mn in Q4 net sales in US
Launched in the US last October, the drug is expected to account for 2% of the country's neutropenia treatment market in 2023
By Feb 02, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


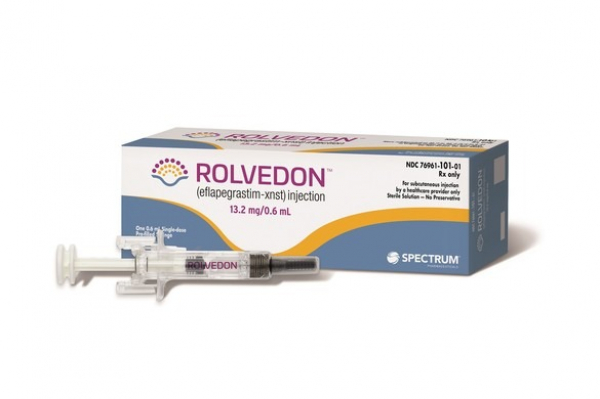
South Korean drugmaker Hanmi Pharmaceutical Co.’s neutropenia agent, Rolvedon (eflapegrastim-xnst), is forecast to achieve about $10 million in net sales in the US for the fourth quarter of 2022.
Spectrum Pharmaceuticals, Hanmi’s sales partner in the US, announced the figure based on preliminary unaudited financial results on Wednesday. The US drugmaker will announce detailed financial updates of the 2022 earnings in March.
Rolvedon, called Rolontis in Korea, was launched in the US last October after it earned a marketing license from the Food and Drug Administration (FDA) in the same year. It is the first new oncology-targeting drug to be developed by a Korean pharmaceutical company and to receive FDA marketing approval.
The neutropenia treatment decreases the incidence of infection in adult patients, who have non-myeloid malignancies and receive myelosuppressive anti-cancer drugs.
Rolvedon is expected to post about $60 million in revenue in the US market this year, accounting for 2% of neutropenia treatment in the country.
The drug achieved a meaningful result during the last quarter in the US neutropenia drug market, where Amgen Inc. is a strong player with Neulasta (pegfilgrastim) and Neupogen (filgrastim), said a Hanmi official.
The Korean biotech will speed up the development of its other new drug candidates by using Lapscovery, its in-house technology that prolongs the residence time of a biologic and minimizes the frequency of treatment, the official said.
Write to Jae-Young Han at jyhan@hankyung.com
Jihyun Kim edited this article.
More to Read
-
 Bio & PharmaSpectrum to push back launch date of Hanmi-developed drug Poziotinib
Bio & PharmaSpectrum to push back launch date of Hanmi-developed drug PoziotinibNov 25, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaHanmi’s anti-cancer drug Rolontis gets FDA approval; US sale via Spectrum
Bio & PharmaHanmi’s anti-cancer drug Rolontis gets FDA approval; US sale via SpectrumSep 13, 2022 (Gmt+09:00)
2 Min read -
 Bio & PharmaFDA to review Hanmi Pharmaceutical’s Rolontis for marketing approval
Bio & PharmaFDA to review Hanmi Pharmaceutical’s Rolontis for marketing approvalApr 13, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaHanmi stands out with own drugs as Korean rivals sell imports
Bio & PharmaHanmi stands out with own drugs as Korean rivals sell importsApr 11, 2022 (Gmt+09:00)
3 Min read
Comment 0
LOG IN


