Bio & Pharma
FDA to review Hanmi Pharmaceutical’s Rolontis for marketing approval
The resubmission of the white blood cell treatment agent comes after the FDA’s remediation request last August
By Apr 13, 2022 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


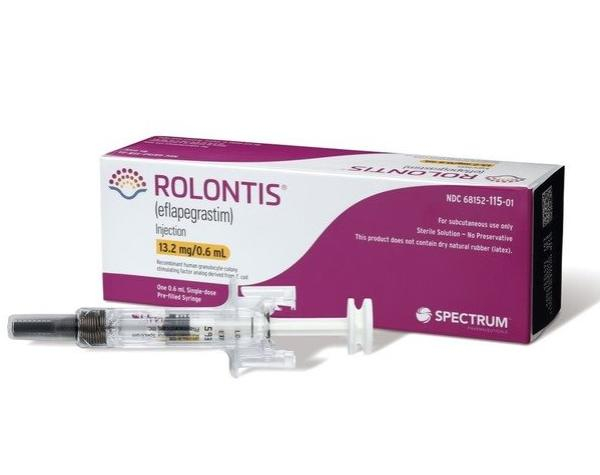
The US Food and Drug Administration has begun reviewing a medicine developed by South Korea’s Hanmi Pharmaceutical Co. for marketing authorization of the white blood cell disease treatment.
The FDA will complete its review of the drug, Rolontis, by Sept. 9 in accordance with the Prescription Drug User Fee Act (PDUFA), Hanmi said on Tuesday.
The US drug administration in August 2021 rejected a previous marketing request by Spectrum Pharmaceuticals Inc., Hanmi’s US partner, for Rolontis, also known as eflapegrastim, citing manufacturing deficiencies at Hanmi’s drug substance facility in Korea.

Spectrum, a US biotechnology firm focused on novel and targeted oncology therapies, recently resubmitted a biologics license application (BLA) to the FDA, which accepted it after remediation of the Hanmi facility.
Spectrum Chief Executive Tom Riga said the FDA’s acceptance of the BLA is important progress in the company’s efforts to bring the agent to the market and that he’s confident that the US drug agency will approve it.
Kwon Se-chang, chief executive of Hanmi, said the company will closely cooperate with Spectrum for the approval and marketing of the drug.
Rolontis, licensed out to Spectrum in 2012, is being developed for the treatment of chemotherapy-induced neutropenia, which occurs when a patient has too few neutrophils, a type of white blood cell. While all white blood cells help the body fight infections, neutrophils are important for fighting certain infections, especially those caused by bacteria.
The molecule of the agent is manufactured by Hanmi at its biomanufacturing site in Korea.
Hanmi, a leading pharmaceutical company in Korea, is also seeking to obtain the FDA’s marketing approval on its lung cancer medicine Poziotinib by the end of this year.
Write to Do-Hee Lee at Tuxi0123@hankyung.com
In-Soo Nam edited this article.
More to Read
-
 Bio & PharmaHanmi stands out with own drugs as Korean rivals sell imports
Bio & PharmaHanmi stands out with own drugs as Korean rivals sell importsApr 11, 2022 (Gmt+09:00)
3 Min read -
 Bio & PharmaKorea Hanmi to launch blockbuster hypertension drug in China
Bio & PharmaKorea Hanmi to launch blockbuster hypertension drug in ChinaMar 11, 2022 (Gmt+09:00)
2 Min read -
 COVID-19War for bio talent intensifies as S.Korea works on mRNA vaccines
COVID-19War for bio talent intensifies as S.Korea works on mRNA vaccinesAug 05, 2021 (Gmt+09:00)
3 Min read -
 Biopharma CMOModerna deal shows Korea’s prowess as global hub for biopharma CMO
Biopharma CMOModerna deal shows Korea’s prowess as global hub for biopharma CMODec 30, 2020 (Gmt+09:00)
3 Min read
Comment 0
LOG IN


