Bio & Pharma
Moderna to stay ahead in mRNA technology: CSO Melissa Moore
The Cambridge, MA-based biotech firm expects its new drug pipeline to significantly increase next year, boosting its sales revenue
By Oct 14, 2022 (Gmt+09:00)
3
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator



CAMBRIDGE, MA – The COVID-19 coronavirus disease has been a curse for many people and companies around the world over the past couple of years. The pandemic, however, had a silver lining for biotechnology companies such as Moderna Inc.
The Massachusetts-headquartered biotech company has become a household name not just in the US but globally after developing one of the most successful vaccines against the disease – a vaccine that uses a copy of a molecule called messenger RNA (mRNA).
With the world’s first bivalent COVID-19 vaccine it developed, Moderna has become the No. 1 company in the mRNA technology segment. Nevertheless, it feels growing pressure to outdo its rivals.
On 200 Technology Square, Cambridge, where the company’s R&D center is located in Greater Boston, some 700 researchers and employees are endeavoring to develop new therapeutics.
“It is actually easier to run fast to catch up to frontrunners. But now we’re the clear frontrunner to catch up to. So, it’s hard to just stay in that position,” Melissa Moore, Moderna's chief scientific officer, said during a recent exclusive interview with The Korea Economic Daily.
“We are aware that there are lots of companies that are trying to catch us and pass us. We need to work even harder.”
HUNT FOR NEW BIOTECH
Following the pandemic, pharmaceutical companies and biotech startups have been accelerating their hunt for next-generation biotechnologies.
On Wednesday, Moderna and Merck & Co. (MSD) agreed to jointly develop a vaccine by combining Moderna’s mRNA tech with Merck’s antibody Keytruda to treat patients with high-risk melanoma, the deadliest form of skin cancer. As part of the deal, Merck will invest $250 million in Moderna.
Earlier this month, France's pharmaceutical giant Sanofi S.A. partnered with US biotech firm miRecule Inc. to develop and commercialize an antibody-RNA conjugate to treat muscular dystrophy.
Moderna’s mRNA vaccine is a new type of vaccine technology.
When used in a vaccine, mRNA is engineered and encoded to activate the body’s immune system to fight the coronavirus. Pfizer Inc., the Boston biotech’s archrival, also produces an mRNA COVID-19 vaccine.
In contrast, traditional vaccines, like those for measles and flu, insert a weakened or inactivated germ into the body to trigger an immune response.
As fears of catching the highly infectious coronavirus disease spread, one of the things that surprised people most was the speed with which Moderna developed its COVID-19 vaccine.
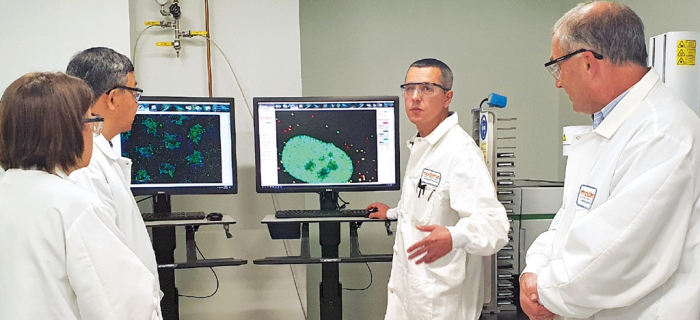
YEARS OF PREPARATION
Before the pandemic, the fastest vaccine developed was the mumps vaccine, which came out after four years of R&D in the 1960s. For a COVID-19 vaccine, it took just 11 months.
Moderna secured a candidate material for clinical trials 45 days after China made public the genome of the first COVID-19 outbreak in Wuhan.
“What people saw was us running the race and how quickly we could do it. What they didn’t see was years of preparation. We had everything in place when COVID-19 arrived,” said Moderna CSO Moore.
Through COVID-19, she said the company learned mRNA vaccines are particularly effective for the elderly population compared to other types of vaccines, boding well for the use of mRNA technology in other geriatric diseases.
Having spent over $300 million annually on R&D efforts for years as an obscure biotech startup, Moderna is now benefiting from its mRNA-based COVID-19 vaccine.
The company made $17.7 billion from its COVID-19 vaccine sales last year. It is expected to post $21 billion in sales this year as it rolls out new booster shots that target the omicron variant.
Moderna has 46 treatment and vaccine candidates, of which 31 have entered clinical trials. Targets for its vaccine candidates include influenza, HIV and respiratory syncytial virus.
The company expects its pipeline to expand to over 100 next year.
Asked about mRNA-based vaccines’ relatively low stability and high price tags, Moore said: “That’s a fair criticism.”
“With any technology, it’s costly when it comes out first. And then over time, the cost comes down and the technology gets perfected, certainly making vaccines more stable,” she said.
Write to Ji-Hyun Lee at bluesky@hankyung.com
In-Soo Nam edited this article.
More to Read
-
 BiotechCelltrion looks beyond biosimilars to develop its own mRNA drugs
BiotechCelltrion looks beyond biosimilars to develop its own mRNA drugsNov 16, 2021 (Gmt+09:00)
4 Min read -
 COVID-19Samsung Biologics-made Moderna vaccine available to Koreans this week
COVID-19Samsung Biologics-made Moderna vaccine available to Koreans this weekOct 27, 2021 (Gmt+09:00)
3 Min read -
 COVID-19War for bio talent intensifies as S.Korea works on mRNA vaccines
COVID-19War for bio talent intensifies as S.Korea works on mRNA vaccinesAug 05, 2021 (Gmt+09:00)
3 Min read -
 Biopharma CMOModerna deal shows Korea’s prowess as global hub for biopharma CMO
Biopharma CMOModerna deal shows Korea’s prowess as global hub for biopharma CMODec 30, 2020 (Gmt+09:00)
3 Min read
Comment 0
LOG IN


