Robotics
Koh Young gets OK from FDA for brain surgery robot
The company aims to expand overseas medical surgery robot business, valued at $12.7 bn
By Jan 21, 2025 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


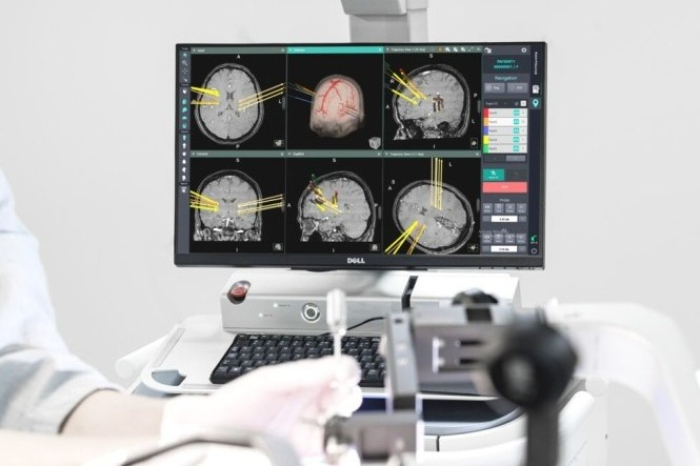
Geniant Cranial is a stereotactic robotic guidance system that uses medical imaging to support complex procedures, including surgeries for epilepsy and Parkinson’s disease, brain tumor biopsies, and intracerebral hemorrhage operations.
Koh Young Technology is the number one company dedicated to developing 3D solder paste inspection (SPI) equipment.
The company developed its first surgical robot, Kymero, in 2011 as part of a Korean government-funded project assigned by the Ministry of Trade, Industry, and Energy. In 2016, the company also received approval from the Ministry of Food and Drug Safety to produce and sell Kymero in South Korea.
After conducting clinical tests in South Korea, Koh Young Technology signed a contract with Yonsei University’s Severance Hospital in June 2020 to supply Kymero for surgical uses.
According to the Korea Institute of Science and Technology Information, the global robot market is expected to more than double to $12.7 billion with North America accounting for 62% by 2025 from $5.9 billion in 2020.
Write to Jong-Hwan Won at won0403@hankyung.com
More to Read
-
 RoboticsKorea’s Koh Young to launch brain surgery robot in US in 2025
RoboticsKorea’s Koh Young to launch brain surgery robot in US in 2025May 09, 2024 (Gmt+09:00)
3 Min read -
 RoboticsKorean 3D tech firm Koh Young looks to US medical robot market
RoboticsKorean 3D tech firm Koh Young looks to US medical robot marketMar 15, 2023 (Gmt+09:00)
2 Min read
Comment 0
LOG IN


