Bio & Pharma
Celltrion applies for interchangeability of Yuflyma in US
The company expects to expand market share through interchangeable prescriptions for the original drug Humira
By Jan 10, 2024 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


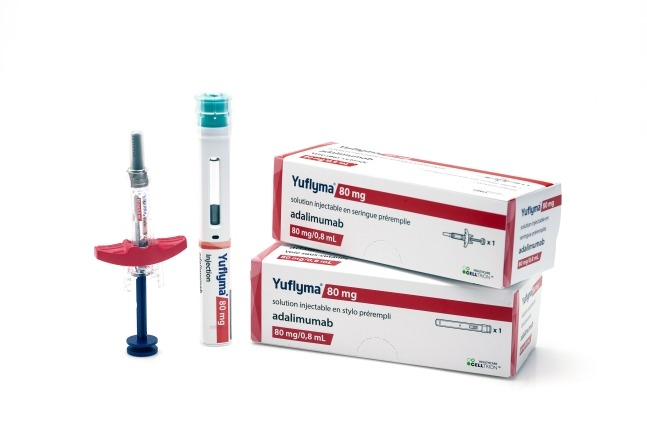
South Korea's Celltrion Inc. announced on Wednesday that it has applied to the US Food and Drug Administration (FDA) for a modified approval to replace its biosimilar Yuflyma with the original treatment Humira for the treatment of autoimmune diseases.
Humira is a blockbuster drug with global sales last year of $21.2 billion (27.4 trillion won), 87% or $18.6 billion of which came from the US. Available in autoinjector and prefilled syringe form, the treatment has secured indications in America for eight diseases including rheumatoid arthritis, Crohn’s disease, and ulcerative colitis.
Yuflyma, a biosimilar developed by Celltrion for Humira, obtained sales approval in the US last year.
If the biosimilar in the US receives approval for interchangeability with the original drug along with the previous product approval, it can be substituted at pharmacies without additional prescriptions from doctors, securing market competitiveness.
Consequently, many biosimilar developers seek clinical trials to confirm interchangeability with the original drugs in addition to clinical trials for approval.
Celltrion had previously demonstrated statistical equivalence between Yuflyma and Humira in a Phase 3 interchangeability clinical trial involving 367 patients with moderate to severe plaque psoriasis.
Celltrion expects that obtaining interchangeability recognition will have a positive impact on expanding Yuflyma's market share in the US.
Write to Jeong Min Nam at peux@hankyung.com
More to Read
-
 Mergers & AcquisitionsCelltrion to sell portfolio of Takeda drugs to CBC Group
Mergers & AcquisitionsCelltrion to sell portfolio of Takeda drugs to CBC GroupJan 01, 2024 (Gmt+09:00)
3 Min read -
 Bio & PharmaCelltrion applies for approval of Xolair biosimilar in Canada
Bio & PharmaCelltrion applies for approval of Xolair biosimilar in CanadaDec 27, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion’s Vegzelma listed on US PBM preferred drug list
Bio & PharmaCelltrion’s Vegzelma listed on US PBM preferred drug listDec 12, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion Healthcare's Herzuma selected as funded brand in NZ
Bio & PharmaCelltrion Healthcare's Herzuma selected as funded brand in NZDec 07, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion applies for approval in Europe for eye treatment biosimilar
Bio & PharmaCelltrion applies for approval in Europe for eye treatment biosimilarNov 24, 2023 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


