Artificial intelligence
Lunit gets FDA approval for AI cancer diagnosis solution
The company’s 3D Breast Tomosynthesis (DBT) AI solution Lunit INSIGHT DBT earns 510(k) clearance, aimimg to enter US market
By Nov 14, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


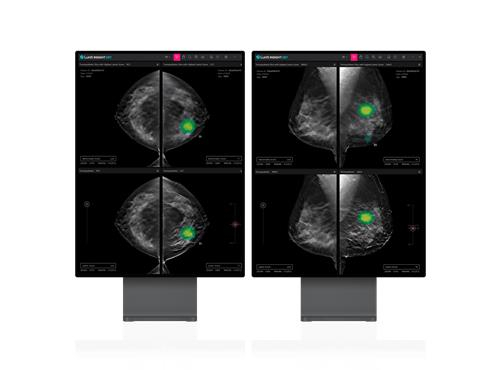
South Korean medical AI company Lunit said on Tuesday its Lunit Insight DBT, an AI imaging diagnosis solution aiding in breast cancer diagnosis, has secured 510(k) pre-market clearance from the US Food and Drug Administration (FDA).
This marks Lunit's third FDA approval, joining its AI emergency triage solution Lunit Insight Triage, and the breast imaging analysis tool Lunit Insight MMG.
The Lunit InsighT DBT analyses 3D images from digital breast tomosynthesis (DBT) with AI, offering faster and more accurate results than traditional 2D mammography.
With FDA approval, Lunit hopes to secure a share of the US market, which represents 65% of global breast cancer screenings, with DBT's widespread adoption.
"We will intensify our efforts in sales and marketing towards US healthcare providers, leveraging our precise AI solutions to swiftly grow our market footprint, responding to the substantial demand for accurate breast cancer diagnostics in the US," CEO of Lunit Suh Beom-Seok said.
Write to Young-Ae Lee at 0ae@hankyung.com
More to Read
-

-
 Bio & PharmaLunit's Insight DBT gets certification from EU for medical device
Bio & PharmaLunit's Insight DBT gets certification from EU for medical deviceMar 20, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaLunit to supply AI image analysis solutions to Singapore
Bio & PharmaLunit to supply AI image analysis solutions to SingaporeMar 09, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaLunit launches AI-based pathology analysis solution with US firm
Bio & PharmaLunit launches AI-based pathology analysis solution with US firmFeb 01, 2023 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


