Beauty & Cosmetics
Cosmax forms dedicated suncare OTC team
It plans to increase its US market share by strengthening its expertise in sunscreens, which are classified as drugs in local
By Oct 12, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


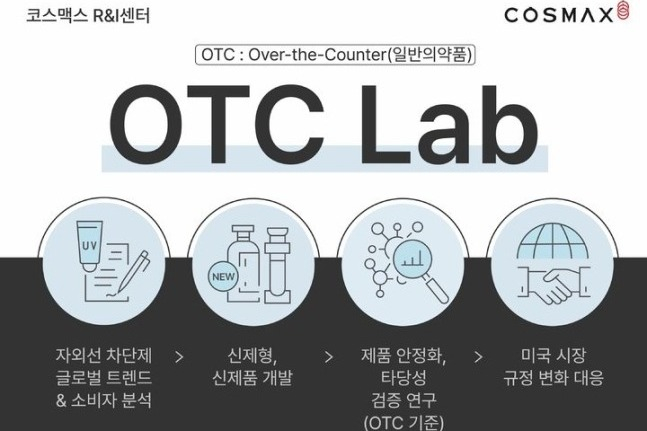
Cosmax Inc., South Korea's largest cosmetics manufacturer that specializes in original design manufacturing (ODM), announced on Thursday the establishment of a dedicated organization for over-the-counter (OTC) sun protection products with UV-blocking functionality within its domestic research and innovation center.
The company intends to proactively respond to the growing demand in the US market for sun care products classified as pharmaceuticals, strengthening its expertise in this field.
Cosmax has obtained approval from the US Food and Drug Administration (FDA) and is manufacturing OTC sun care products in both its South Korean factory and its US subsidiary in New Jersey.
In the US, sunscreens and other UV blockers, which are classified as pharmaceuticals, are subject to FDA regulations and oversight at the pharmaceutical level, unlike in South Korea, where they are classified as functional cosmetics. Sunscreens, as well as acne treatments, dandruff shampoos and others, fall under the category of OTC products in the US.
Manufacturing or repackaging any pharmaceutical products for use within the United States requires FDA registration for the facilities involved. Therefore, the standards applied for sunscreen production are stringent, as they are assessed according to pharmaceutical standards, not cosmetic ones, unlike in South Korea.
Lately, there has been an increasing demand in the US sun care market for multifunctional sunscreen products that include skincare benefits, and dermatology and independent brands are on the rise.
The performance and user experience of so-called "K-sunscreens" have gained recognition on online video platforms and other media, leading to increased attention to Korean brands. Consequently, there is a growing demand for beauty companies aiming to penetrate the US market with "Made-in-Korea" sunscreens.
Leveraging its accumulated expertise, Cosmax will actively support its clients in the early stages of entering the US OTC sun care market and will continuously expand its range of sunscreen formulations and product offerings that meet OTC requirements.
Write to Kyeong-je Han at hankyung@hankyung.com
More to Read
-
 Beauty & CosmeticsS.Korea's Cosmax wins halal award in Indonesia
Beauty & CosmeticsS.Korea's Cosmax wins halal award in IndonesiaSep 20, 2023 (Gmt+09:00)
2 Min read -
 Beauty & CosmeticsCosmax opens Asia's largest cosmetics plant in China
Beauty & CosmeticsCosmax opens Asia's largest cosmetics plant in ChinaAug 14, 2023 (Gmt+09:00)
1 Min read -
 Beauty & CosmeticsCosmax extracts raw materials from indigenous plants in Indonesia
Beauty & CosmeticsCosmax extracts raw materials from indigenous plants in IndonesiaJul 24, 2023 (Gmt+09:00)
1 Min read -
 Beauty & CosmeticsCosmax to make inroads into Japanese market in earnest
Beauty & CosmeticsCosmax to make inroads into Japanese market in earnestJan 19, 2023 (Gmt+09:00)
1 Min read -
 Beauty & CosmeticsCosmax shows off advanced beauty tech at CES with Seoul Nat'l Univ.
Beauty & CosmeticsCosmax shows off advanced beauty tech at CES with Seoul Nat'l Univ.Jan 10, 2023 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


