Bio & Pharma
FDA suspends COVID-19 diagnostic kit of S.Korea’s SD Biosensor
The US watchdog cites danger of bacterial infection and is mulling a recall
By May 08, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


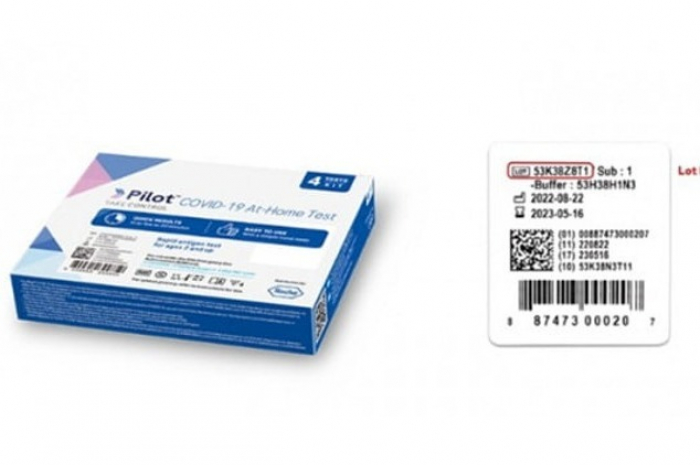
The US Food and Drug Administration (FDA) has recommended that consumers discard select models of Pilot COVID-19 self-diagnostic test kits sold on the American market by South Korea's SD Biosensor Inc..
In a notice on Thursday, the watchdog warned that the solution in the kit may be infected with bacteria and released 44 lot (production process) numbers of the items in question.
Contaminated products could result in misleading diagnoses such as false negatives or positives, the FDA said, and it can cause disease in people with weak immunity. If symptoms of fever, conjunctivitis or other systemic bacterial infections appear in a user of the kit, immediate medical attention was recommended.
“The FDA has not received reports of injuries, adverse health consequences, or death associated with use of the SD Biosensor Pilot COVID-19 At-Home Test to date,” it added.
CVS Health and Amazon have distributed about 516,000 of the pilot tests in the US, the notice said. SD Biosensor in 2021 received emergency approval for the kit’s use and has sold it on the American market through the global medical device company Roche Diagnostics.
The FDA is also mulling the kit’s recall, saying, “The FDA is currently reviewing the SD Biosensor Pilot COVID-19 At-Home Tests recall and is in the process of classifying the recall risk. The FDA is continuing to work with SD Biosensor Inc. to assess the company’s corrective actions to address the reason for bacterial contamination and help ensure the situation is resolved and will not return.”
Write to Hyun-Ah Oh at 5hyun@hankyung.com
More to Read
-
 Bio & PharmaKorea's SD Biosensor to supply diagnostics kits to Japan in H2
Bio & PharmaKorea's SD Biosensor to supply diagnostics kits to Japan in H2Aug 18, 2022 (Gmt+09:00)
2 Min read -
 Mergers & AcquisitionsSD Biosensor seeks more M&As, to build diagnostic kit plant in US
Mergers & AcquisitionsSD Biosensor seeks more M&As, to build diagnostic kit plant in USJul 10, 2022 (Gmt+09:00)
3 Min read -
 Mergers & AcquisitionsSD Biosensor, SJL Partners to acquire Meridian Bioscience for $1.53 bn
Mergers & AcquisitionsSD Biosensor, SJL Partners to acquire Meridian Bioscience for $1.53 bnJul 07, 2022 (Gmt+09:00)
5 Min read -
 COVID-19COVID-19 blues? Pandemic makes SD Biosensor Korea’s top-grossing bio firm
COVID-19COVID-19 blues? Pandemic makes SD Biosensor Korea’s top-grossing bio firmJul 01, 2021 (Gmt+09:00)
3 Min read -
 Mergers & AcquisitionsCOVID test kit maker SD Biosensor buys Italian medical device firm
Mergers & AcquisitionsCOVID test kit maker SD Biosensor buys Italian medical device firmApr 22, 2022 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


