Bio & Pharma
Celltrion applies for FDA approval for Remsima SC
The autoimmune disease treatment has seen positive results from a global phase 3 clinical trial
By Dec 23, 2022 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


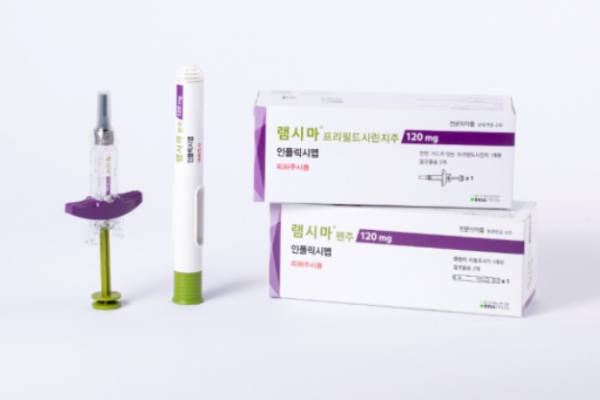
Celltrion Ltd. said on Friday it had applied the previous day for permission from the US Food and Drug Administration (FDA) for Remsima SC, an autoimmune disease treatment.
Remsima SC (infliximab) is a self-injectable drug developed as a subcutaneous injection formulation.
A Celltrion official said it applied to the FDA based on results confirming the efficacy and safety of the global phase 3 clinical trial for ulcerative colitis and Crohn's disease.
"The influence of Remsima SC is rapidly increasing with a market share of more than 12% in Europe, where it entered the market earlier, thanks to its rapid dosing effect and convenience of formulation," said a Celltrion official.
"Once it secures new drug status in the US, it will further strengthen the position of the Remsima family and provide more patients with high-quality drug treatment opportunities," he added.
Write to Jae-Young Han at jyhan@hankyung.com
More to Read
-
 Bio & PharmaCelltrion to seek FDA approval for sale of Remsima SC by year-end
Bio & PharmaCelltrion to seek FDA approval for sale of Remsima SC by year-endNov 28, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion wins trial against Regeneron for US patent lawsuit over biosimilar
Bio & PharmaCelltrion wins trial against Regeneron for US patent lawsuit over biosimilarNov 18, 2022 (Gmt+09:00)
1 Min read -
 EarningsCelltrion's Q3 earnings boosted by Remsima sales in Europe
EarningsCelltrion's Q3 earnings boosted by Remsima sales in EuropeNov 09, 2022 (Gmt+09:00)
1 Min read -

Comment 0
LOG IN


