Pharmaceuticals
HK inno.N’s K-CAB a blockbuster drug with $843 million exports
Kolmar Korea’s acquisition of the company is touted as one of its most successful deal stories
By Dec 24, 2021 (Gmt+09:00)
2
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


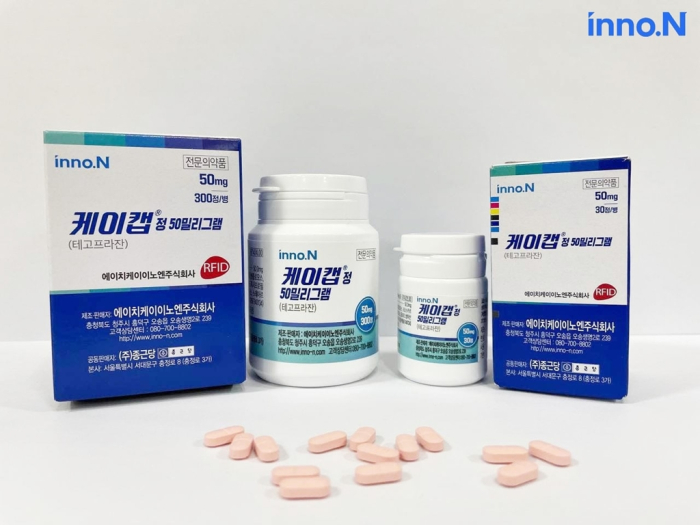
South Korea’s HK inno.N Corp. has signed a 640 billion won ($540 million) contract to license out its technology for K-CAB, a gastroesophageal reflux disease drug, to Braintree Laboratories Inc., a subsidiary of US-based Sebela Pharmaceuticals Inc.
The deal, the Korean company’s single largest contract for K-CAB Tab., will bring the drug’s total export value to 1 trillion won ($843 million) since its launch in 2019, setting the stage for the medicine to become a global blockbuster.
Under the contract, HK inno.N will receive a down payment of $2.5 million, technology fees for each clinical and licensing progress stage, and royalties according to sales, the company said on Thursday.
The contract term for K-CAB, a potassium competitive acid blocker (P-CAB) product, is 15 years after sales begin in the US market. Currently, another treatment for gastroesophageal reflux disease (GERD), known as proton pump inhibitors (PPIs), is the only type available in the US.

HK inno.N, a pharmaceutical unit of cosmetics firm Kolmar Korea, said it will also supply raw materials for K-CAB to Braintree Laboratories.
With the latest contract, the company’s global exports of K-CAB have reached about 1 trillion won, including $95 million technology exports to China, $84 million to 17 Latin American countries, including Mexico, and 200 billion won to six Southeast Asian countries, including Indonesia.
A GREAT BARGAIN
When Kolmar Korea acquired CJ HealthCare, the original developer of K-CAB Tab., for 1.31 trillion won in February 2018, industry officials said the price tag was too high, given that the deal was valued at 20 times Kolmar Korea’s annual revenue.
However, the acquisition, led by Kolmar Korea Vice Chairman Yoon Sang-hyun, has become one of the company’s biggest success stories with K-CAB set to become a global blockbuster drug.
Following the acquisition, CJ HealthCare was renamed HK inno.N Corp.

Designated as Korea’s 30th novel drug in 2019, K-CAB (active ingredient: tegoprazan) has since been selling well, as it gained attention as a next-generation therapeutic agent thanks to its advantages such as rapid drug efficacy and overcoming limitations of conventional PPI-based products -- the most widely prescribed drugs for GERD.
HK inno.N’s sales and profits have been steadily rising, helped by K-CAB, hypertension treatment drug Herben, and CONDITION, one of Korea’s top-selling hangover remedies.
The company went public through an initial public offering on the Kosdaq market in August to secure the funds needed to develop more drugs and expand globally.
A company official said a total of 16 new drugs are in the pipeline.
Write to Sang-Hun Oh at ohyeah@hankyung.com
In-Soo Nam edited this article.
More to Read
-

-
-
 IPOsHK inno.N’s IPO price set at top end with record demand for Kosdaq listing
IPOsHK inno.N’s IPO price set at top end with record demand for Kosdaq listingJul 28, 2021 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


