Biotech
Korea’s MedPacto, Merck plan phase 3 trials of colorectal cancer drug
The deal marks the first time for a Korean biotech company to work with Merck in phase 1-3 collaboration
By Dec 15, 2021 (Gmt+09:00)
2
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator



MedPacto Inc., a South Korean biotechnology company that develops therapeutics for cancer and autoimmune diseases, has agreed with global drugmaker Merck & Co Inc. to jointly conduct phase 3 clinical trials of their respective cancer treatments in combination.
Under the agreement, Merck will supply Keytruda, also known as pembrolizumab, an antibody used in cancer immunotherapy, to MedPacto free of charge for global phase 3 studies to evaluate the safety and efficacy of the Korean firm’s Vactosertib, a colorectal cancer treatment candidate.
The clinical trials, to be initiated in early 2022 and completed by 2026, will include approximately 500 to 600 patients at about 40 sites in the US and South Korea, MedPacto said on Tuesday.
The patients will be treated with a combined regimen of Vactosertib and Keytruda, compared with physicians’ choice of standard of care, the company said.
The collaboration follows promising results from the phase 1 and 2 clinical trials, for which Merck also provided MedPacto with Keytruda for free.
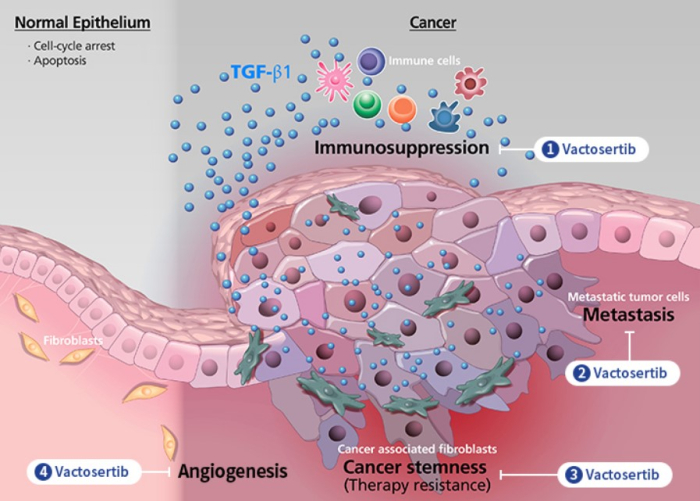
CLOSER TO NEW DRUG APPROVAL
“This marks the first time that a Korean biotech company has collaborated with Merck from phases 1 through 3 in clinical tests,” MedPacto said in a statement.
Merck, also known as MSD outside the US and Canada, is involved in about 1,700 cases of Keytruda-related clinical trials globally, of which only 13 cases are in the phase 3 stage.
In June, MedPacto released the interim results of its phases 1 and 2 clinical trials using a combination treatment of Vactosertib and Keytruda for colorectal cancer patients.
The Korean company said at the time that the combination resulted in median overall survival (mOS) of 15.8 months – a promising improvement compared to the current approved standard regimen with an mOS of fewer than seven months.

Citing data from BioMedtracker, a US clinical trial monitoring firm, MedPacto said Vactosertib’s entry into the phase 3 stage has significantly increased its possibility of obtaining approval from the FDA and other drug agencies.
“We hope that this trial will support our submission of a new drug application,” said MedPacto founder and Chief Executive Kim Seong-jin.
Write to Ju-Hyun Lee at deep@hankyung.com
In-Soo Nam edited this article.
More to Read
-

-
 COVID-19 treatmentSK seeks to make Pfizer, Merck COVID-19 pills in CMO deals
COVID-19 treatmentSK seeks to make Pfizer, Merck COVID-19 pills in CMO dealsNov 17, 2021 (Gmt+09:00)
3 Min read -
 COVID-19 treatmentCelltrion wins COVID-19 treatment approval from Europe
COVID-19 treatmentCelltrion wins COVID-19 treatment approval from EuropeNov 15, 2021 (Gmt+09:00)
4 Min read
Comment 0
LOG IN


