COVID-19
COVID-19 blues? Pandemic makes SD Biosensor KoreaŌĆÖs top-grossing bio firm
The company is set to go public this month to raise money for business expansion
By Jul 01, 2021 (Gmt+09:00)
3
Min read
Most Read

Multinational biopharmaceutical companies have dominated the worldŌĆÖs diagnostics market for years. However, the coronavirus pandemic, which started early last year, has offered an unexpected boost for smaller South Korean test-kit makers, getting their names to the global arena and allowing them to make a decent profit.
One such company is SD Biosensor Inc., which posted a mediocre operating profit of 1.5 billion won ($1.3 million) on revenue of 72.9 billion won in 2019, just a year before the COVID-19 breakout.
In 2020, the company posted a 492-fold surge in operating profit to 738.3 billion won from the previous year. Sales picked up to 1.7 trillion won, a 23-fold jump.
Its revenue and profit growth have accelerated into this year with no conspicuous signs of COVID-19 cases abating.
In the first quarter of this year, SD Biosensor, now the countryŌĆÖs top test kit maker, racked up its largest-ever quarterly results with an operating profit of 576.3 billion won on sales of 1.2 trillion won, buoyed by strong demand for coronavirus test kits.
With its first-quarter operating profit, SD Biosensor became the highest-grossing company among KoreaŌĆÖs biopharmaceutical companies, earning seven times more than that of market leader Samsung Biologics Co. Of all listed Korean firms, just 16 companies were ahead of SD Biosensor in profit ranking.
Industry watchers attributed SD BiosensorŌĆÖs significant growth to its aggressive facility investment and price competitiveness.
The company sold about 700 million units of COVID-19 test kits globally from February 2020 to May this year, becoming the worldŌĆÖs top test kit maker by volume.
It started developing the coronavirus test kit in early January last year, about half a month before the emergence of KoreaŌĆÖs first infected case.
In February 2020, it obtained approval of its COVID test kit from KoreaŌĆÖs Ministry of Food and Drug Safety. By September, the company won the World Health OrganizationŌĆÖs emergency use authorization, the first such permit by the WHO.
SPEEDY DECISION-MAKING
Cho Young-shik, chairman and founder of SD Biosensor, has been in the diagnostic field since 1984.
With his 10-year experience at GC Pharma Corp., better known as Green Cross Corp., as an R&D researcher, he launched his own diagnostic company, SD, in 1999. The company produced various kinds of rapid testing kits for illnesses such as HIV, Dengue and Malaria.
In 2009, SD was acquired by US firm Alere Inc. via a hostile M&A. But two years later, he took over the test kit division from the US diagnostic company and established a new entity, which has grown into SD Biosensor.
ŌĆ£As soon as COVID-19 broke out in China last year, we spent billions of won to establish a mass production system for test kits even without any product orders,ŌĆØ said Chairman Cho. ŌĆ£Such speedy decision-making made us what we are today.ŌĆØ
IPO IN MID-JULY
In the first quarter, rapid test kits accounted for over 90% of its revenue. Its antigen-based diagnostic kit takes less than half an hour to determine if a person is positive for the virus, but its accuracy hovers around 80% to 90%.
The company is now developing a new product, a real-time polymerase chain reaction (PCR) testing kit, to continue its growth.
It plans to unveil the product next month following seven years of research and development.
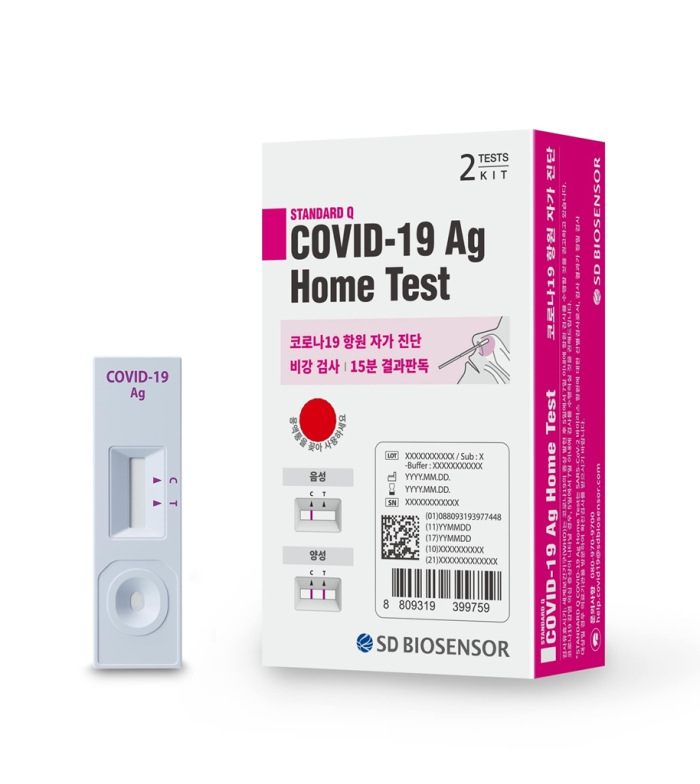
However, SD Biosensor said its PCR test product takes less than an hour while maintaining test result accuracy at 99%. The test kit can also be used to detect 11 diseases, including Dengue, AIDS and flu.
To raise money for business expansion, the company plans to go public on the main Kospi market as early as this month.
In May, SD Biosensor said it applied for an initial public offering to raise up to 1.3 trillion won ($1.2 billion) with its corporate value estimated at about 6 trillion won.
The diagnostic reagent maker plans to offer its shares, including 10 million new issues, at between 66,000 and 85,000 won.
Its bookbuilding with institutional investors is scheduled for July 5-6 with retail subscription due for July 8-9.
NH Investment & Securities Co. and Korea Investment & Securities Co. are joint book runners.
Write to Woo-Sub Kim and Ju-Hyun Lee at duter@hankyung.com
In-Soo Nam edited this article.
More to Read
-
 BiotechKoreaŌĆÖs new PCR kits, devices to speed COVID-19 detection
BiotechKoreaŌĆÖs new PCR kits, devices to speed COVID-19 detectionJul 15, 2021 (Gmt+09:00)
2 Min read -

-
 Korean chipmakersChip shortage hits KoreaŌĆÖs COVID-19 diagnostic device manufacturers
Korean chipmakersChip shortage hits KoreaŌĆÖs COVID-19 diagnostic device manufacturersJun 07, 2021 (Gmt+09:00)
3 Min read -
Comment 0
LOG IN




