EC
Scope
Date
~
-
 Bio & Pharma
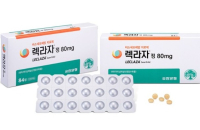
Bio & PharmaYuhan’s lung cancer drug gets OK in Europe
Leclaza (ingredient: lazertinib), a new lung cancer treatment developed by South Korea’s Yuhan Corp. secured approval from the European Commis...
Dec 31, 2024 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaSamsung Bioepis gets EC's approval for Opuviz
South Korea's Samsung Bioepis Co., Samsung Biologics Co.'s fully owned biosimilar subsidiary, and the US-based Biogen said on Tuesday the European C...
Nov 19, 2024 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaSamsung Bioepis gets OK to sell Stelara biosimilar in Europe
South Korea's Samsung Bioepis has received approval from the European Commission to sell Pyzchiva, a biosimilar of the autoimmune disease Stelara.&n...
Apr 23, 2024 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion gains sales approval for biosimilar Vegzelma in Europe
Celltrion Healthcare Co., South Korean biopharmaceutical giant Celltrion Inc.'s global marketing affiliate, said on Friday it has obtained the Europ...
Aug 19, 2022 (Gmt+09:00)
-
 COVID-19
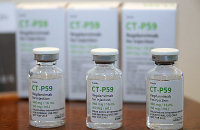
COVID-19Celltrion wins COVID-19 treatment approval from Europe
South Korea’s pharmaceutical giant Celltrion Inc. has won final approval for its COVID-19 treatment Regkirona from the European Commission, a ...
Nov 15, 2021 (Gmt+09:00)
language
Latest News
- 1 Seoul appeal: Korean art captivates Indonesia’s affluent connoisseurs
- 2 CJ CheilJedang scraps $3.5 bn green bio sale, shifts gears to expansion
- 3 Trump Jr. meets Korean business chiefs in back-to-back sessions
- 4 Samsung in talks to supply customized HBM4 to Nvidia, Broadcom, Google
- 5 Kookmin Bank raises $700 mn in forex bonds amid strong demand
