CT-P17
Scope
Date
~
-
 Bio & Pharma
Bio & PharmaCelltrion unveils cancer medicine to expand new drug pipelines
South Korea’s leading biosimilar drug developer Celltrion Inc. on Sunday unveiled an anticancer medicine to expand new pipelines and accelerat...
Apr 28, 2025 (Gmt+09:00)
-
 Bio & Pharma
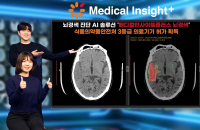
Bio & PharmaSK C&C wins Korea’s nod for brain infarct diagnosis AI solution
SK Inc C&C (SK C&C), the information technology service unit of South Korea’s SK Group, said on Thursday it has secured medical device...
Feb 22, 2024 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion applies for approval of Xolair biosimilar in Canada
South Korean biopharmaceutical company Celltrion Inc. announced on Wednesday that it has applied for CT-P39, a biosimilar of the treatment of asth...
Dec 27, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion applies for approval in Europe for eye treatment biosimilar
South Korean biopharmaceutical company Celltrion Inc. announced on Friday that it applied for approval from the European Medicines Agency (EMA) for ...
Nov 24, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion to release Stelara biosimilar in US from March 2025
South Korean biopharmaceutical company Celltrion Inc. announced on Friday that it has completed a patent agreement with Johnson & Johnson, the m...
Aug 25, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion gets OK partial approval for phase 3 of biosimilar in Europe
South Korean biopharmaceutical company Celltrion Inc. announced on Tuesday that it has received approval from the European Medicines Agency (EMA) fo...
Aug 22, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion wins phase 3 IND approval for Ocrevus biosimilar in US
South Korea's biopharmaceutical company Celltrion Inc. announced that it received on Thursday approval from the US Food and Drug Administration (FDA...
Jun 15, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion unveils phase 1 clinical data of Actemra biosimilar in Europe
South Korean biosimilar giant Celltrion Inc. revealed on Wednesday the highly anticipated clinical phase 1 data for its Actemra biosimilar, CT-P47, ...
May 31, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion gets FDA approval for Humira biosimilar CT-P17
South Korean biopharmaceutical company Celltrion Inc. announced on Wednesday that it has obtained product approval from the US Food and Drug Adminis...
May 24, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion applies for Phase 3 clinical trials in Europe for biosimilar
South Korea’s Celltrion Inc. is speeding up global clinical trials of its biosimilar of the multiple sclerosis (MS) treatment Ocrevus (active ...
May 03, 2023 (Gmt+09:00)
-
 Bio & Pharma
Bio & PharmaCelltrion wins trial against Regeneron for US patent lawsuit over biosimilar
South Korean biopharmaceutical company Celltrion Inc. announced Friday that it won its first trial against New York-based biotech giant Regeneron Ph...
Nov 18, 2022 (Gmt+09:00)
Latest News
- 1 Seoul appeal: Korean art captivates Indonesia’s affluent connoisseurs
- 2 CJ CheilJedang scraps $3.5 bn green bio sale, shifts gears to expansion
- 3 Trump Jr. meets Korean business chiefs in back-to-back sessions
- 4 Samsung in talks to supply customized HBM4 to Nvidia, Broadcom, Google
- 5 Kookmin Bank raises $700 mn in forex bonds amid strong demand
