Middle East: New land of opportunity for Korean medical devices
The region could be a springboard for expansion into Europe and Africa
By Feb 10, 2025 (Gmt+09:00)
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund



South Korea is making inroads into the Middle East's medical device market dominated by the US and Europe, aiming to leverage the region’s strong purchasing power and big investments in building digital hospital infrastructure.
Korean developers of disease treatment, diagnostics and medical aesthetic devices are capitalizing on the global popularity of Korean culture and beauty products, as well as the region’s population growth.
With the Middle East relying on imports for more than 90% of medical devices, Korean companies are outshining Chinese peers, whose appeal has faded amid escalating trade rows between the US and China.
Last month, Remed Co., LaMeditech Co., Vuno Inc., Mediwhale Co. and Neurophet Inc. exhibited their products at Arab Health, a healthcare conference held in Dubai. It is the world’s largest healthcare device trade show.
During the conference, Korean medical device makers bagged a total of $4.17 million in supply contracts with companies from around 40 countries.
LaMeditech secured a large distributor in the Middle East for its skin disease treatment device Careveam at 2025 Arab Health.

The medical device market of Saudi Arabia and the United Arab Emirates (UAE) combined is forecast to expand to $3.95 billion in 2026, according to industry officials.
During the Medlab Middle East Global In Vitro Diagnostics Exhibition and the UAE International Dental Conference & Arab Dental Exhibition, Korean diagnostics developers such as SD Biosensor Inc., NGeneBio Co. and Sugentech Inc. showcased their products. The two exhibitions took place early this month.
“Over 1,000 visitors stopped by our booth for two days, showing strong interest in our products,” said a Sugentech official. The company opened a booth at the Medlab Middle East Global In Vitro Diagnostics Exhibition.
“We’re in serious discussions with more than 50 distributors.”
Lunit Inc. and Deepnoid Inc. have already exported AI-powered diagnostics devices to the Middle East, driven by the region’s push for smart hospitals.

Medical aesthetic device companies are also scurrying to secure their toehold in the Middle East. Won Tech Co. started selling Oligio, its flagship skin-lifting laser machine, in Dubai in November. Last month, the skin tightening machine maker obtained approval for an additional six products in the UAE.
QUALITY AND PRICES
According to the Korea Customs Service, four Middle Eastern countries – Saudi Arabia, UAE, Qatar and Kuwait – imported $79.2 million worth of medical devices from South Korea in 2024, up 88% from 2021.
“Thanks to their high quality and affordable prices, the demand for Korean medical devices is growing in the Middle East with the high income and strong purchasing power,” said a medical device industry official.
Once they establish their presence in the Middle East, they can further expand into Europe and Africa.
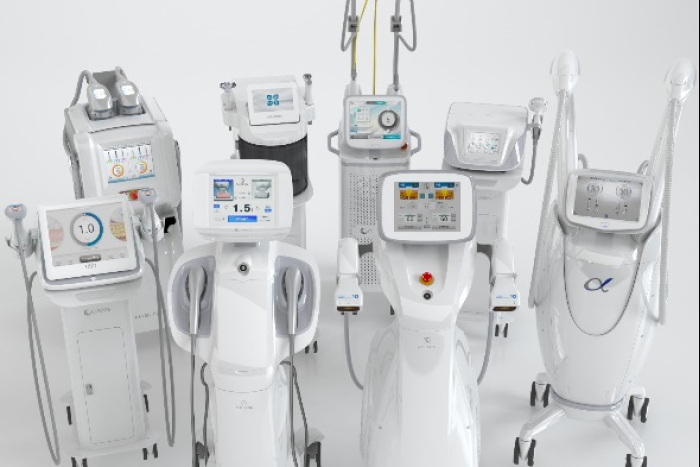
ENTRY BARRIERS
The Middle East still presents high barriers to medical device startups.
To qualify for exports to Saudi Arabia, medical devices must first obtain approval from the US, Japan and Europe. That would make it challenging for newcomers to target the Middle East as their first overseas market.
Write to Hyun-Ah Oh at 5hyun@hankyung.com
Yeonhee Kim edited this article.
-
 Private equityFrance's Archimed seeks to delist aesthetic laser maker Jeisys
Private equityFrance's Archimed seeks to delist aesthetic laser maker JeisysJun 10, 2024 (Gmt+09:00)
1 Min read -
 Bio & PharmaNGeneBio gets approval for cancer diagnostic device in Thailand
Bio & PharmaNGeneBio gets approval for cancer diagnostic device in ThailandDec 22, 2023 (Gmt+09:00)
1 Min read -
 Korean startupsKorean AI startups Allganize, Neurophet raise over $35 mn
Korean startupsKorean AI startups Allganize, Neurophet raise over $35 mnNov 08, 2023 (Gmt+09:00)
2 Min read -

-
 Artificial intelligenceVuno's brain imaging AI medical device secures FDA certification
Artificial intelligenceVuno's brain imaging AI medical device secures FDA certificationOct 10, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaNGeneBio applies for US patent of early diagnosis tech of dementia
Bio & PharmaNGeneBio applies for US patent of early diagnosis tech of dementiaSep 14, 2023 (Gmt+09:00)
1 Min read -
 Artificial intelligenceNeurophet strives to be first mover in fight against Alzheimer’s
Artificial intelligenceNeurophet strives to be first mover in fight against Alzheimer’sMay 24, 2023 (Gmt+09:00)
3 Min read -
 Bio & PharmaLunit's Insight DBT gets certification from EU for medical device
Bio & PharmaLunit's Insight DBT gets certification from EU for medical deviceMar 20, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaMedical IP's X-ray image analysis software certified as medical device
Bio & PharmaMedical IP's X-ray image analysis software certified as medical deviceMar 08, 2023 (Gmt+09:00)
1 Min read -
 Artificial intelligenceVuno's fundus-reading AI obtains medical device certification in Thailand
Artificial intelligenceVuno's fundus-reading AI obtains medical device certification in ThailandDec 15, 2022 (Gmt+09:00)
1 Min read -
 Mergers & AcquisitionsCOVID test kit maker SD Biosensor buys Italian medical device firm
Mergers & AcquisitionsCOVID test kit maker SD Biosensor buys Italian medical device firmApr 22, 2022 (Gmt+09:00)
1 Min read


