Future Unicorns
Future Unicorns

AIOBIO is a healthcare technology startup that is revolutionizing the dental industry with focus on early diagnosis and preventative care. Its quantitative light-induced fluorescence (QLF™) technology and Q-ray devices allow easy check on oral health condition in both clinics and at home. Its dental service and solutions embrace disease prevention, diagnosis, treatment as well as post-treatment maintenance stages.
- Story
- Data
Leading a Dental Paradigm Shift in Korea
Away from Mere Treatment towards Holistic Prevention
Most people say that dental treatments are too costly. But such claim is not totally accurate. It only applies to patients suffering from severe tooth decay and who therefore need a dental implant or a crown after a root canal. Visits to dentists are expensive when people neglect their oral health. Expensive dental treatments aren’t needed if people check their oral hygiene on a regular basis.
In South Korea, people rarely think they need dental checkups and believe dental appointments are necessary only when there is perceptible pain or an evident issue. Prevention and checkups account for only 10% of total dental payments in Korea, whereas the portion is much higher at 70% in the US.
AIOBIO is a healthcare startup with a vision to become a total dental service and solutions provider embracing disease prevention, diagnosis, treatment as well as post-treatment maintenance. In the vision of AIOBIO founder and CEO Yoon Hong-cheol: “We are not only providing our self-invented dental diagnostic equipment to dental clinics in and outside Korea, but also paving the way to develop an AI-based, personalized home dental healthcare service. And even further, we are looking to establish partnerships with global companies and expand internationally.”
Contents
-
[Starting Point] An Opportune Meeting with a Dutch Physicist
Yoon is a dentist with a doctorate in dental medicine from Yonsei University in Korea. He has been in practice since he opened his own clinic in the middle of the Asian Financial Crisis in 1998. Reflecting on his decision to expand on his practice and start a separate business venture, Yoon said “a meeting with a Dutch physicist nine years ago was the event that really led me into the world of bio-health.” That physicist is Dr. Elbert de Josselin de Jong, now serving as a technical advisor at AIOBIO’s research center.
Dr. de Jong is the inventor of QLF™ (Quantitative Light-induced Fluorescence) technology. When de Jong was doing his PhD in micro-radiography at the University of Groningen in the 1980s, he discovered that he could use light to identify dental decay. With his breakthrough, de Jong started to look for business opportunities, starting in 1993 at a dentistry conference in Germany where he introduced the prototype of what is now the signature QLF™ device, combining diagnostic equipment with medical software.
With the prototype, de Jong ran his first series of clinical tests in the US in 1996, received FDA approval in 2004, and launched the product accordingly. But market reaction was muted. Only 200 units were sold. Not only was the size of the device too large, the price was also too high. As a result, most of the units were used for research rather than clinical purposes.
But as fate would have it, in 2005, Dr. de Jong had a meeting that would change everything. At a dentistry conference in Osaka, Japan, a renowned Korean professor showed keen interest in the QLF™ technology. During the conference, Professor Kim Baek-il of Yonsei University College of Dentistry and Dr. de Jong talked day and night about advancing the technology. With de Jong moving to Korea two years later, they worked together on various research projects with a successful market launch of the QLF™ diagnostic dental device as their common goal.
Kim introduced Yoon to de Jong as a possible business partner when he thought their technology was good enough for commercialization in Korea. Yoon was operating his own clinic in the Gangnam area at the time. During their first meeting, Yoon and de Jong found a kindred spirit and agreed to co-establish AIOBIO in 2011.
Yoon’s long-term ambition is to make AIOBIO QLF™ products the industry norm: “Just as you take X-rays when you have issues with your bones or muscles, people will be seeking diagnoses through our devices when they have issues with their teeth.” -
[Technology] Early Diagnosis of Dental Decay with Blue Light Technology
As part of the QLF™ system, the Q-ray camera can examine dental plaque, tartar, decay, fissures and other dental problems that are difficult to identify with the naked eye. The Q-ray uses 405-nanometer visible blue light as opposed to radioactive rays used in X-rays.
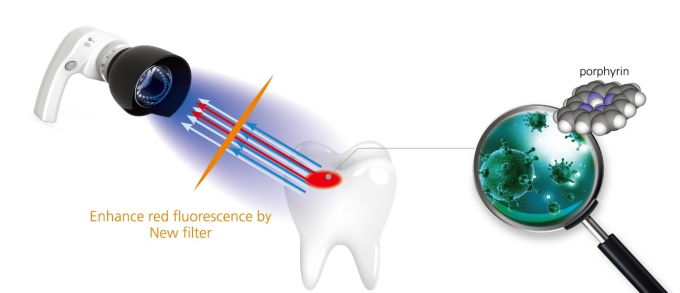
QLF technology enhances red fluorescence with a new type of filter; porphyrin is a material that creates visible fluorescence
The QLF™ device utilizes the simple physics principle of wavelength difference in identifying the damage. Specifically, when the blue light is shone on teeth, healthy enamel and damaged enamel reflect the light at different wavelengths. Once the reflected light is examined using special film, only the damaged part displays bright red light.
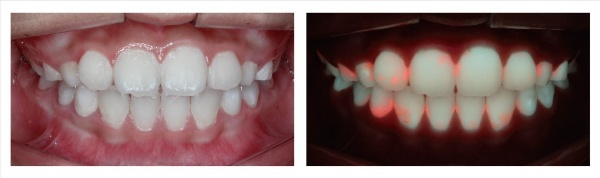
How QLF™ works in comparison to ordinary photography
Yoon said, “If a dental symptom can be identified through a dentist’s ocular inspection or based on X-ray results, then it means the teeth have already been severely damaged. Our QLF™ product can identify dental symptoms at very early stages and can manage them before it’s too late.”
AIOBIO’s commercial launch of QLF™ was not without its challenges. The company failed in its first attempt to gain approval from the Korean government’s New Health Technology Assessment, a regulatory hurdle every company must pass to commercialize its technology and launch the product in the market.
“Companies often complain that the New Health Technology Assessment is too difficult to pass. We understand that from failing in our first try. I hope our case is a benchmark for other companies,” said Yoon.
After receiving obligatory approval from the Ministry of Food and Drug Safety, AIOBIO again applied for the New Health Technology Assessment but was rejected due to lack of sufficient clinical test results verifying the effectiveness and safety of its devices.
From that point it took almost five years of hard work to pass the assessment in August 2018 as the first approval ever for such a dental diagnostic device under the new assessment system. Between its first and second attempts, AIOBIO had conducted nine clinical tests with Yonsei University and published two articles in a relevant academic journal. Reflecting back, Yoon admitted that his company’s original application lacked full preparation and emphasized, “it is critical to have sufficient data to prove your product’s technological excellence.”
AIOBIO has also developed its own software utilizing device data to quantify the state of a patient’s oral hygiene. The software can categorize the level of tooth decay from Q0 to Q3 and plaque from 0 to 5, for better understanding of the exact state of a patient’s dental health. -
[Value Proposition] Gum Disease and Cavities also Chronic Diseases
According to Korea’s Health Insurance Review and Assessment Service (HIRA), the national agency responsible for reviewing national insurance payments, gum disease and tooth decay ranked first and fourth, respectively, in terms of the number of patients. There were 16.7 million gum disease patients and 6.5 million tooth decay patients last year, both exceeding the 6.3 million high blood pressure patients. This is why Yoon says, “dental diseases are chronic diseases representative of our population.”
Yoon further highlighted that our teeth and gum become “extremely vulnerable once they become damaged and thus need longer periods of treatment. That’s exactly why we need to take preventative measures.”
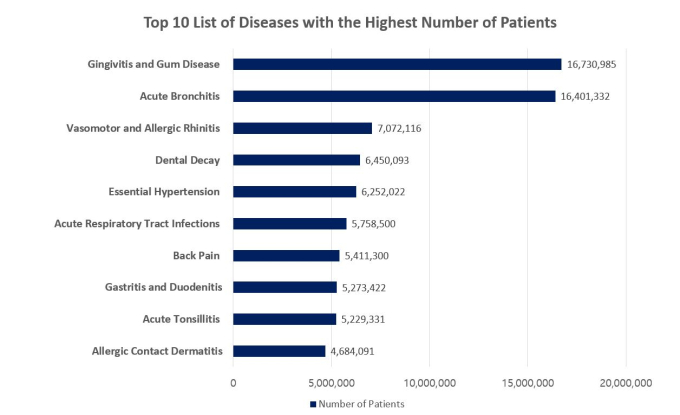
Source: National Statistics on Most Frequent Diseases in 2019 (HIRA); Out-patient Data Only
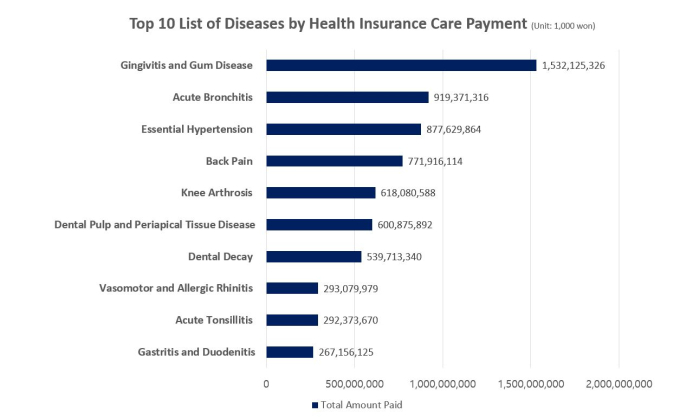
Source: National Statistics on Most Frequent Diseases in 2019 (HIRA); Out-patient Data Only
AIOBIO has built a dental disease treatment system called 5 Step Dentistry in 2018 after its QLF™ methodology passed the government’s New Health Technology Assessment. The system is called ‘5 Step Dentistry’ as it is made up of five stages of dental care: screening, diagnosis, assessment, treatment and post-treatment maintenance.
Yoon said that dentists have a tendency to focus on the third and fourth stages, stressing that his company’s vision is to expand dental care service also into the screening, diagnosis and maintenance stages.
AIOBIO has launched eight medical devices so far, including its flagship product, Qraycam Pro. The company also sells healthcare devices for household use in addition to professional medical devices for hospitals and clinics. According to Yoon, many recent government projects in the bio and health sectors stress the importance of early diagnosis technology. He explained that his company’s timely focus on dental diagnosis technology was a major factor in the company’s success in recent years.
“We need to start regarding dental diseases as chronic diseases that need lifelong care, and also categorize dentists as internal medicine doctors – just as in Sweden and some other countries in Europe. Koreans visit dentists only when we feel pain but that’s not the case in Europe. We need a system that can provide regular care for dental patients at each stage,” said Yoon. -
[Revenue Model] Creating New Demand in Both B2B and B2C Channels
AIOBIO’s revenue model is mainly twofold. The first is the B2B part, targeting hospitals and clinics, while the second part of the business targets consumers directly. Yoon believes that additional revenue will be generated in the dental industry if diagnosis becomes a more established practice. Currently, diagnosis accounts for very little of overall dental industry revenue.
AIOBIO said that it is meaningful that the company is driving diagnosis sector growth, even if the diagnosis segment expands as little as 10% from current levels. Yoon said, “10% may sound like nothing, but what is important is that we are creating a new market segment.”
AIOBIO launched its first QLF™ medical device Qraycam Pro in the domestic market in 2014, and now more than 800 hospitals and clinics globally are using the diagnostic camera. There are 18,000 dental hospitals and clinics in Korea, and AIOBIO targets supplying its devices to 3,600 of them, 20% of the total, by 2022.
AIOBIO’s QLF™ device has also passed the review by the South Korean health authorities for coverage by national health insurance. According to the health ministry’s administrative notice in May 2021, patients aged 5 to 12 can receive public health insurance benefits every six months from June 2021 for dental check-ups that use the QLF™ devices such as Qraypen C.
“The patients only have to pay around 4,000 won ($3.59) per dentist visit to check whether they have tooth decay,” said AIOBIO CEO.
Yoon also claims that AIOBIO’s QLF™ devices will cut dental costs in other aspects as well. “Early diagnosis of dental decay with our equipment will prevent the patient from needing root canal treatments, therefore drastically reducing dental treatment costs. Such a system will benefit the patients, hospitals, clinics and even the national health insurance service.”
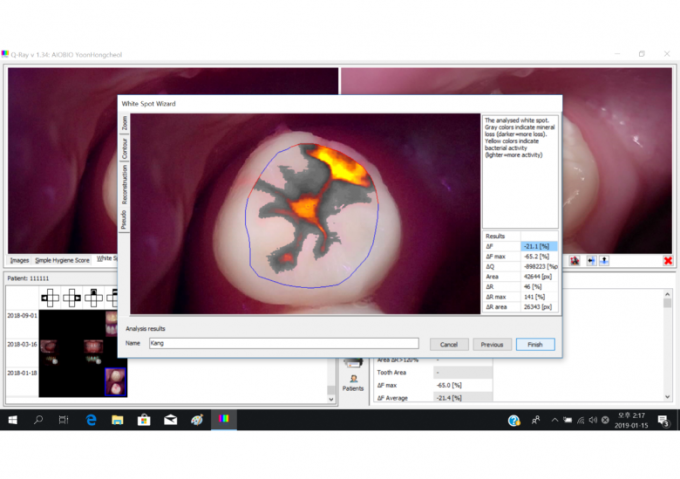
AIOBIO’s Q-ray software measuring four categories: ΔF, ΔF max, ΔR, ΔR max
Individual households are also potential customers of AIOBIO. The company’s QLF™ devices have a large potential for expansion outside hospitals and clinics as they are easy to use at home and safe because they use visible light rather than radioactive rays. As with the recent popularity of LED masks for skincare at home, AIOBIO believes it will be at the vanguard of home-based oral care.
To make its dental service more holistic, AIOBIO is also creating a mobile app that anyone can use to check their oral hygiene synced with accumulated data using QLF™ devices. AIOBIO’s app project is receiving funding from the Ministry of Science and ICT, with clinical tests planned in 2021. Based on preliminary sales of home-version QLF™ devices and after solidifying the app’s monetization model, the company aims to secure 2,000 household customers by 2022. Achieving such a target will generate an additional 300 million won for the company in the household healthcare service segment.
Yoon said “if people can check their oral health more easily and thus more frequently, they could discover issues that require a trip to the dentist to get the situation under control – ultimately having a positive impact on the profitability of dental clinics.” -
[Funding] So Far 6 Billion Won, and headed for 2023 Kosdaq Listing
In 2019, AIOBIO had issued 4.5 billion won worth of RCPS (redeemable convertible preference shares), which were purchased by investment firms including DSC Investment, Korea Investment Partners and IMM Investment. Among the investors, DSC Investment stands out as a VC with highly successful investment track record in the bio sector; previous investments include those to ABL Bio, SCM Life science and Genome & Co. DSC Investment had already invested 1.5 billion won on AIOBIO in 2016 and carried out another series of investments last year.
AIOBIO also raised funds from about 30 individual investors. These investors are medical professionals who give technical advice and who have the potential to expand their business partnerships. Yoon said that the company will “raise additional funding in Q4 this year after discussing with our investors,” adding that AIOBIO plans to be listed on the Kosdaq in 2023 through a special listing channel for technology companies. -
[Scalability] Building Big AI and Data-Powered Dental Healthcare Platform with Global Ambition
Yoon stated that AIOBIO has now entered an acceleration stage, scaling up its international presence and drastically expanding its business model. In the short run, the company will primarily focus on making their QLF™ technology more prevalent both in and outside Korea. Yoon said that his company’s next step would be collaboration with global oral care product companies to “create a comprehensive dental service that combines our personal diagnostic equipment with more traditional oral care products such as toothpaste and toothbrushes.”
He added that B2B sales would be another focus, saying “we will expand sales to dental clinics and hospitals by raising awareness of our brand and technology.” AIOBIO currently exports its dental equipment to five countries, namely Australia, India, Mexico, Russia and The Netherlands, and plans to expand further into other regions.
The company said that “while the current environment for overseas sales in not too favorable due to COVID-19, we target around 20% of total revenue will be generated outside Korea by 2021.” The company aims to receive product approval from the US and European authorities by the end of this year.
In the long run, AIOBIO aims to build a digital platform on dental healthcare for individuals. Using AI technology and big data, the platform will enable patients to self-manage dental data and take more ownership of their oral health. Yoon commented that the company would continue to receive funding totaling 2.9 billion won from the Ministry of Health and Welfare until 2023 on the digital platform project.
Yoon is confident that the government funding and public-private partnerships will usher in a new era of business growth. “More accurate and precise treatment can be delivered if we combine our technology and equipment with the government-led project My Data, which will allow patients to autonomously use their own medical data for various purposes.”
Q-Ray Products
By Yu Yim; edited by Daniel Cho (freeu@hankyung.com)