Bio
Celltrion to co-develop pill-type autoimmune disease drug with UK startup
By Aug 20, 2020 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


Biosimilar maker Celltrion Inc. has agreed to develop a tablet-type treatment for autoimmune diseases jointly with the UK biotech startup Intract Pharma, the South Korean company said on August 20, a move seen as an effort to expand its presence in the monoclonal antibody biosimilar market.
Both companies will develop a pill version of infliximab, the main ingredient of Celltrion’s monoclonal antibody biosimilar Remsima.
The agreement comes after Celltriont developed the world’s first intravenous injection and subcutaneous (SC)-injection types of Remsima, a biosimilar version of Janssen’s Remicade which is also a brand name of infliximab.
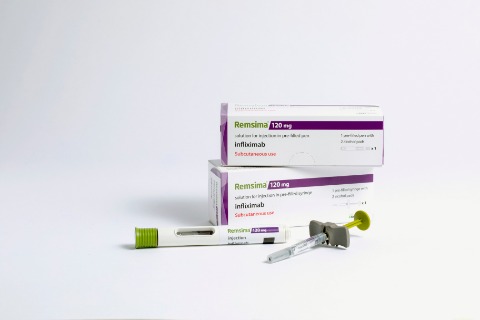
Celltrion controls more than half the market of biosimilar versions of Remicade in Europe. The recent introduction of the SC-injection type is expected to help the Korean biosimilar maker increase its market share, given that Janssen and other biosimilar makers have not yet developed the SC-injection type of infliximab.
Under the agreement, Celltrion will supply its infliximab biosimilar as a raw material for clinical trials to Intract Pharma. The UK biotech company will be responsible for the tablet-type development and clinical tests, using its oral immunotherapy technology designed to push the molecules of a drug absorbed into the blood through the intestinal wall.
Celltrion will also secure priority rights over the new development upon completion of second-phase clinical trials. If they transfer the technology to a third company, Celltrion will receive part of the sales as royalties.
Additionally, Celltrion will supply its infliximab biosimilar exclusively to the third company when it succeeds in commercializing the oral-version treatment, using their technology.
Both companies are exempted from first-phase non-clinical and clinical tests for the oral infliximab. Intract Pharma will kick off second-phase clinical trials next year for patients with inflammatory bowel diseases.
Write to Woo-sub Kim at duter@hankyung.com
Both companies will develop a pill version of infliximab, the main ingredient of Celltrion’s monoclonal antibody biosimilar Remsima.
The agreement comes after Celltriont developed the world’s first intravenous injection and subcutaneous (SC)-injection types of Remsima, a biosimilar version of Janssen’s Remicade which is also a brand name of infliximab.

Celltrion controls more than half the market of biosimilar versions of Remicade in Europe. The recent introduction of the SC-injection type is expected to help the Korean biosimilar maker increase its market share, given that Janssen and other biosimilar makers have not yet developed the SC-injection type of infliximab.
Under the agreement, Celltrion will supply its infliximab biosimilar as a raw material for clinical trials to Intract Pharma. The UK biotech company will be responsible for the tablet-type development and clinical tests, using its oral immunotherapy technology designed to push the molecules of a drug absorbed into the blood through the intestinal wall.
Celltrion will also secure priority rights over the new development upon completion of second-phase clinical trials. If they transfer the technology to a third company, Celltrion will receive part of the sales as royalties.
Additionally, Celltrion will supply its infliximab biosimilar exclusively to the third company when it succeeds in commercializing the oral-version treatment, using their technology.
Both companies are exempted from first-phase non-clinical and clinical tests for the oral infliximab. Intract Pharma will kick off second-phase clinical trials next year for patients with inflammatory bowel diseases.
Write to Woo-sub Kim at duter@hankyung.com
Yeonhee Kim edited this article
More to Read
-
 Business & PoliticsTrump Jr. meets Korean business chiefs in back-to-back sessions
Business & PoliticsTrump Jr. meets Korean business chiefs in back-to-back sessionsApr 30, 2025 (Gmt+09:00)
-
 Korean chipmakersSamsung in talks to supply customized HBM4 to Nvidia, Broadcom, Google
Korean chipmakersSamsung in talks to supply customized HBM4 to Nvidia, Broadcom, GoogleApr 30, 2025 (Gmt+09:00)
-
 EnergyLS Cable breaks ground on $681 mn underwater cable plant in Chesapeake
EnergyLS Cable breaks ground on $681 mn underwater cable plant in ChesapeakeApr 29, 2025 (Gmt+09:00)
-
 Business & PoliticsUS tariffs add risk premium to dollar assets: Maurice Obstfeld
Business & PoliticsUS tariffs add risk premium to dollar assets: Maurice ObstfeldApr 29, 2025 (Gmt+09:00)
-

Comment 0
LOG IN


