SK Biopharm submits NDA of epilepsy drug to China
The company got the $15 mn milestone payment after handing in NDA to China's NMPA
By Dec 05, 2024 (Gmt+09:00)
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


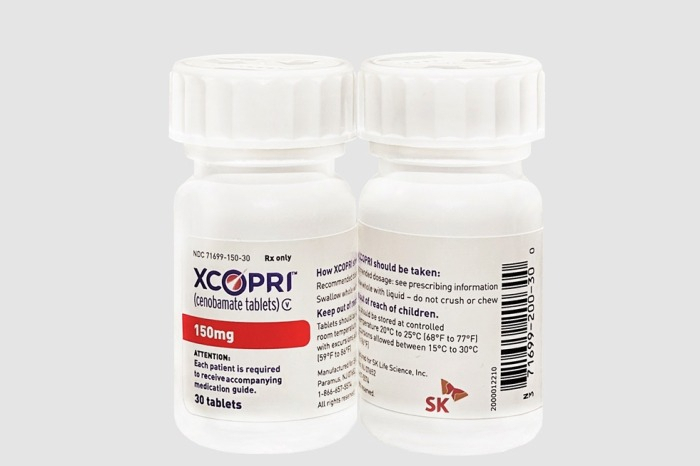
South Korea's SK Biopharmaceuticals Co. said on Tuesday it submitted the new drug application (NDA) for its flagship epilepsy drug Cenobamate to China's National Medical Products Administration (NMPA) through Ignis Therapeutics, the joint venture of SK Biopharmaceuticals and 6D Capital.
The company won the $15 mn milestone payment after handing in the NDA to NMPA.
China is the world's largest market for epilepsy drugs. In China, there are around 10 million people with epilepsy.
SK Biopharmaceuticals' Cenobamate has entered the US and European markets. The company also aims to expand into Greater China.
Write to Dae-Kyu Ahn at powerzanic@hankyung.com
-

-
 Korean chipmakersSamsung in talks to supply customized HBM4 to Nvidia, Broadcom, Google
Korean chipmakersSamsung in talks to supply customized HBM4 to Nvidia, Broadcom, Google21 HOURS AGO
-
 EnergyLS Cable breaks ground on $681 mn underwater cable plant in Chesapeake
EnergyLS Cable breaks ground on $681 mn underwater cable plant in ChesapeakeApr 29, 2025 (Gmt+09:00)
-
 Business & PoliticsUS tariffs add risk premium to dollar assets: Maurice Obstfeld
Business & PoliticsUS tariffs add risk premium to dollar assets: Maurice ObstfeldApr 29, 2025 (Gmt+09:00)
-



