Bio & Pharma
Korea’s Lunit in AI-powered lung cancer diagnostics deal with AstraZeneca
The deal marks the first time for a Korean medical AI firm to partner with a global pharma for Al-based lung cancer diagnosis
By Nov 18, 2024 (Gmt+09:00)
2
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


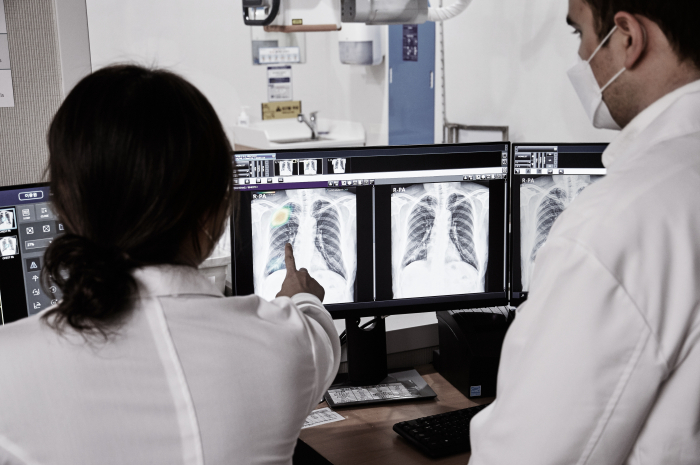
Lunit Inc., a South Korean AI-powered cancer diagnostics company, has partnered with global biopharma giant AstraZeneca plc. to jointly enter the AI-powered lung cancer diagnostics market.
Ken Nesmith, Lunit's chief business officer, said in an interview with The Korea Economic Daily that the Korean firm has been picked as AstraZeneca’s sole partner for using AI technology to identify patients eligible for administration of Tagrisso, the biopharma's lung cancer medicine.
The partnership marks the first time a Korean medical AI firm has teamed up with a global pharmaceutical company to enter the AI-powered lung cancer diagnosis market.
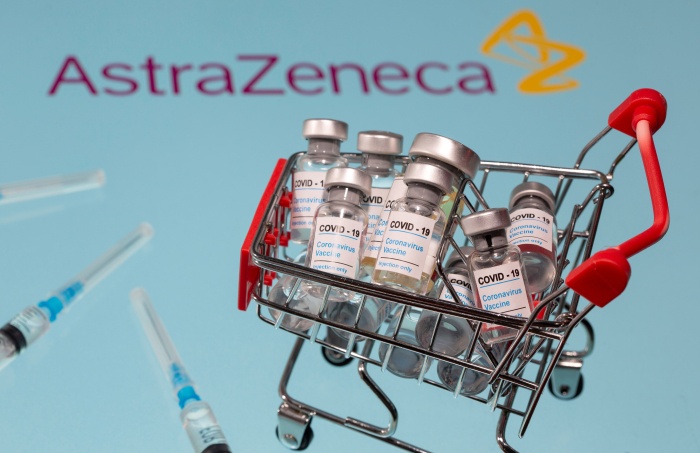
On Monday, the two companies signed a formal strategic business cooperation agreement.
“In recent competitive bidding, Lunit was chosen as the sole AI biomarker partner for non-small cell lung cancer (NSCLC). Lunit’s partnership with an oncology leader like AZ is a testament to our cutting-edge AI technology,” said Nesmith.
Tagrisso is a treatment for NSCLC, a condition that accounts for 85% of lung cancer cases globally. About 700,000 lung cancer patients worldwide are using the drug.
AZ’S TAGRISSO
About half of NSCLC cases occur in patients with a mutation in the epidermal growth factor receptor (EGFR) gene. Tagrisso is effective for lung cancer patients with this mutation.
The key challenge lies in identifying patients with EGFR mutations before treatment. If Tagrisso is prescribed to patients without the mutation, it is ineffective, which leads to unnecessary medical expenses and delayed treatment opportunities for patients, officials of the companies said.
For this reason, the need to correctly diagnose patients with EGFR mutations has steadily risen, even among big pharmaceutical firms such as AZ, which ranked seventh globally with 64 trillion won ($48 billion) in revenue in 2023.
LUNIT’S AI BIOMARKER
Pharmaceutical companies have traditionally used a test method called next-generation sequencing (NGS) to detect genetic mutations.
These tests, however, are costly and time-consuming.
Lunit said its EGFR mutation detection AI biomarker, Lunit SCOPE Genotype Predictor, analyzes digitized tissue slide images and delivers results within five minutes.
The speedy test results and accuracy were key to its deal with AZ, it said.
“Our AI technology enables rapid mutation predictions, allowing physicians to prioritize necessary NGS tests for eligible patients, enhancing efficiency. This also reduces cases of prescribing unsuitable drugs, a persistent issue in current medical practices,” said Nesmith.
Lunit and AZ are considering expanding their cooperation on the use of AI-powered diagnostics to detect other types of lung cancer.
Lunit Chief Executive Brandon Suh said in July the company will accelerate its push into developing markets grappling with inadequate cancer diagnostic technology and infrastructure.
In May, Lunit acquired Volpara Health Technologies Ltd., a New Zealand-based AI software developer, to broaden its presence in the breast cancer detection market.
Write to Jeong Min Nam at peux@hankyung.com
In-Soo Nam edited this article.
More to Read
-
 Artificial intelligenceLunit eyes developing markets with AI cancer diagnostics
Artificial intelligenceLunit eyes developing markets with AI cancer diagnosticsJul 09, 2024 (Gmt+09:00)
4 Min read -
 Mergers & AcquisitionsLunit buys breast cancer detection AI firm Volpara Health
Mergers & AcquisitionsLunit buys breast cancer detection AI firm Volpara HealthMay 22, 2024 (Gmt+09:00)
2 Min read -
 Korean startupsHealthQuest-backed Lunit applies for 2022 Kosdaq IPO
Korean startupsHealthQuest-backed Lunit applies for 2022 Kosdaq IPONov 30, 2021 (Gmt+09:00)
3 Min read -
 Korean startupsHealthQuest picks Korea’s Lunit for first investment in Asia
Korean startupsHealthQuest picks Korea’s Lunit for first investment in AsiaNov 24, 2021 (Gmt+09:00)
2 Min read
Comment 0
LOG IN


