JLK gets FDA OK for prostate cancer diagnosis AI solution
It will boost expanding into the US market with its Medihub Prostate can MR images and prostate-specific antigen
By Jun 25, 2024 (Gmt+09:00)
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


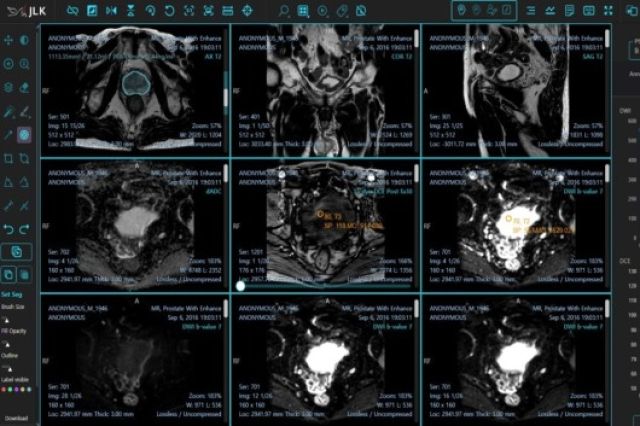
South Korea’s JLK Inc., an AI-based diagnosis solution and platform maker, announced on Monday that it got approval from the US Food and Drug Administration (FDA) for its prostate cancer diagnostic solution Medihub Prostate.
The solution aids prostate cancer diagnosis by analyzing prostate magnetic resonance (MR) images and assessing prostate-specific antigen (PSA) levels.
Prostate cancer is the most common cancer among men in the US, with a latent prostate cancer occurrence risk of up to 40%.
"Based on the FDA approval of MEDIHUB Prostate, we plan to pursue entry into the US market more aggressively," JLK's CEO Kim Dong-min said.
"We intend to sequentially apply for FDA approval for three additional AI solutions between August and October."
Write to Jeong Min Nam at peux@hankyung.com
-

-
 Bio & PharmaKorea's JLK targets AI-based stroke care market in US
Bio & PharmaKorea's JLK targets AI-based stroke care market in USJan 10, 2024 (Gmt+09:00)
2 Min read -