Bio & Pharma
Celltrion'a autoimmune disease drug Zymfentra makes US debut
The Korean drugmaker aims for more than $750 million in revenue from the new drug by 2025
By Mar 18, 2024 (Gmt+09:00)
1
Min read
Most Read
Alibaba eyes 1st investment in Korean e-commerce platform


Blackstone signs over $1 bn deal with MBK for 1st exit in Korea


OCI to invest up to $1.5 bn in MalaysiaŌĆÖs polysilicon plant


Korea's Lotte Insurance put on market for around $1.5 bn


NPS loses $1.2 bn in local stocks in Q1 on weak battery shares



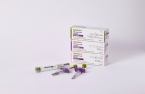
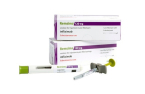
South Korean biopharmaceutical giant Celltrion Inc. said on March 17 that it has launched its new autoimmune disease treatment Zymfentra in the US market.
The drug is a subcutaneous injection of CelltrionŌĆÖs infliximab Remsima, a treatment for adults with ulcerative colitis or CrohnŌĆÖs disease. It won US Food and Drug Administration approval last October.
The drug blocks the action of tumor necrosis factor-alpha (TNF-alpha), a protein that can be overproduced and cause the immune system to attack certain parts of the body. A patient can self-administer the medicine subcutaneously without needing to visit a medical center, Celltrion said.
Celltrion said it expects Zymfentra to have long-term profitability in the US as the drug will be under patent protection through 2040.
The drugmaker aims for more than 1 trillion won ($749.9 million) in revenue from Zymfentra by 2025, exceeding 10% of prescriptions for the target diseases in the US market. The wholesale price of Zymfentra, for two doses administered for four weeks, is set at $6,181.1.
The company said some pharmacy benefit managers (PBMs) in the US have registered the medicine to their formularies so that the drugs can be covered by prescription plans.
The TNF-alpha market in the US reached 62.1 trillion won in 2022, including inflammatory bowel diseases (IBDs) ŌĆō ulcerative colitis and CrohnŌĆÖs disease ŌĆō for 12.8 trillion won, Celltrion cited healthcare market research firm IQVIAŌĆÖs data. Infliximab is the most common drug for IBDs, Celltrion added.
ŌĆ£The novel subcutaneous administration represents an important advancement in patient care that can offer a convenient treatment option, allowing patients in the US to have greater flexibility in managing their disease,ŌĆØ said Celltrion USA Chief Commercial Officer Thomas Nusbickel.
Write to Dae-Kyu Ahn at powerzanic@hankyung.com
┬Ā
Jihyun Kim edited this article.
More to Read
-
-
-
 Bio & PharmaCelltrion Holdings eyes Nasdaq IPO by early 2025: chairman
Bio & PharmaCelltrion Holdings eyes Nasdaq IPO by early 2025: chairmanJan 16, 2024 (Gmt+09:00)
1 Min read -
 Bio & PharmaCelltrion revises up earnings goals to overtake Amgen
Bio & PharmaCelltrion revises up earnings goals to overtake AmgenJan 11, 2024 (Gmt+09:00)
3 Min read
Comment 0
LOG IN


