Bio & Pharma
CGBio gets FDA approval for Novosis Putty
The S.Korean company plans to apply the US clinical trials of composite bone graft material in H1 2024
By Jan 02, 2024 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


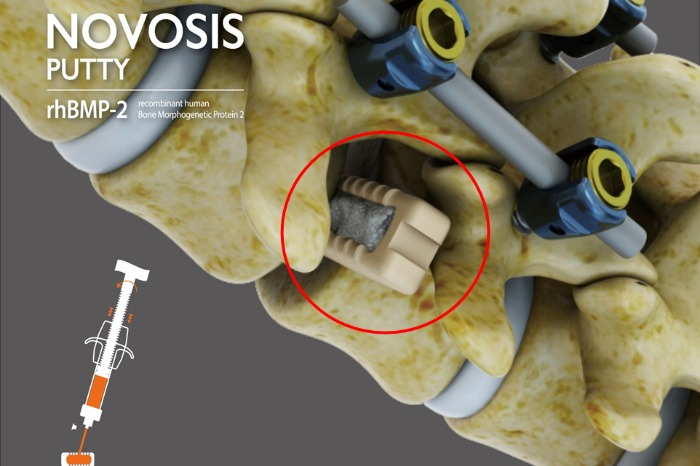
South Korea's bio-regenerative medical firm CGBio announced on Tuesday that its Novosis Putty has been designated as a breakthrough device by the US Food and Drug Administration (FDA).
This marks the first case of an implantable medical device developed by a South Korean company receiving FDA breakthrough device designation.
FDA's breakthrough device designation is a program designed to expedite the entry of groundbreaking medical technologies into the market. It provides benefits such as priority in the US approval process, flexible clinical trial design, and close support from a dedicated review team.
Novosis Putty is a bone substitute containing recombinant human bone morphogenetic protein (rhBMP-2). When bones are damaged, it aids in the differentiation of stem cells within the human body into bone cells, facilitating the formation of new bone.
CGBio plans to apply for US confirmation clinical trials to demonstrate the safety and efficacy of Novosis Putty in the first half of this year.
Write to Yoo-Rim Kim at youforest@hankyung.com
More to Read
-
 Bio & PharmaS.Korea's CGBio gets FDA approval for spinal implant
Bio & PharmaS.Korea's CGBio gets FDA approval for spinal implantAug 14, 2023 (Gmt+09:00)
1 Min read -
 Beauty & CosmeticsS.Korea's CGBio exports $8 mn filler to Australia, New Zealand
Beauty & CosmeticsS.Korea's CGBio exports $8 mn filler to Australia, New ZealandMar 24, 2023 (Gmt+09:00)
1 Min read -
 Beauty & CosmeticsCGBio opens cosmetic surgery clinic in Bali using bio-medical tech
Beauty & CosmeticsCGBio opens cosmetic surgery clinic in Bali using bio-medical techMar 14, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaCGBio inks export deal for hyaluronic acid fillers with Chinese distributor
Bio & PharmaCGBio inks export deal for hyaluronic acid fillers with Chinese distributorMar 22, 2023 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


