SK Biopharm gears up to boost epilepsy drug sales in Europe
In 2024, it will embark on the process of winning FDA approval for an epileptic seizure detection device
By Nov 16, 2023 (Gmt+09:00)
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator



SK Biopharmaceuticals Co. is gearing up to explore the European market for its flagship epilepsy drug Cenobamate, while looking for new mergers and acquisition targets to sharpen its competitiveness in the seizure treatment market, said its Chief Executive Lee Donghoon.
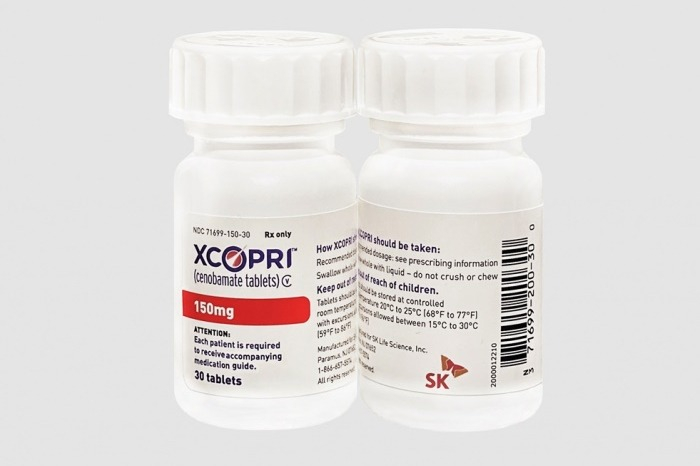
Cenobamate is its first commercialized drug. It has seen its market share in the US grow since receiving the green light from the US Food and Drug Administration (FDA) in 2019.
SK sells it under the brand name Xcopri, for which it recently extended its sales rights by four years to the end of October 2032.
“Cenobamate has built its presence in the US with annual sales of over 200 billion won ($153 million), but it is in its infancy stage in Europe,” Lee told The Korea Economic Daily in a recent interview.
It was his first media interview as SK Pharmaceuticals’ CEO after he took the position early this year.
“We’re also carrying out clinical trials in Europe to expand the indications for Cenobamate,” he said. The company is trying to apply the drug to a broader range of symptoms than those for which it was originally intended.
In Europe, it sells Cenobamate in 23 countries through its partner Angelini Pharma.
SK Biopharmaceuticals’ Estimated 2023 Earnings
Unit: Billion won
Note: Fourth-quarter results are estimates
Source: SK Biopharmaceuticals
M&As
In August, it completed the buyout of Proteovant Therapeutics, a US biotechnology company with protein degradation technology, to reinforce its targeted protein degradation (TPD) platform for 62 billion won.
It is now looking to buy a central nervous system (CNS) drug candidate through an M&A.
“We will likely launch our second commercial drug for CNS diseases after Cenobamate around 2025 to 2026,” Lee said at a press conference in July this year.
MEDICAL DEVICE
Next year, SK Biopharmaceuticals will embark on the process of obtaining approval from the FDA for a medical device that detects epileptic seizures.
“We want to diversify our distribution channels in preparation for the expansion of our products.”
As its new growth engines, he picked radiopharmaceuticals (RPT), targeted proteolytic therapy (TPD) and cell and gene therapy (CGT). They are viewed as next-generation anti-cancer treatments.
“From a new drug development perspective, the fastest one is TPD,” he said. “We have discovered a protein decomposition substance and are developing it as a targeted anti-cancer drug.”
In line with its efforts to build its presence in Europe, Lee presented his plan to nurture new growth engines such as RPT at the Jefferies 2023 London Healthcare Conference this week. Jefferies is Europe's largest pharmaceutical and bio-investment conference.
Write to Jeong Min Nam at peux@hankyung.com
Yeonhee Kim edited this article.
-
 Bio & PharmaSK Biopharma’s Cenobamate US patent extended by 2032
Bio & PharmaSK Biopharma’s Cenobamate US patent extended by 2032Nov 10, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharm to export epilepsy drug tech to Middle Eastern firm
Bio & PharmaSK Biopharm to export epilepsy drug tech to Middle Eastern firmAug 18, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharm eyes Asia's No.1 spot in radioactive drug market
Bio & PharmaSK Biopharm eyes Asia's No.1 spot in radioactive drug marketJul 19, 2023 (Gmt+09:00)
2 Min read -
 Bio & PharmaS.Korea’s SK to buy US bio JV stake from Roivant for $48 mn
Bio & PharmaS.Korea’s SK to buy US bio JV stake from Roivant for $48 mnJun 30, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharm’s Cenobamate gets nod in Canada, to boost US sales
Bio & PharmaSK Biopharm’s Cenobamate gets nod in Canada, to boost US salesJun 15, 2023 (Gmt+09:00)
2 Min read -
 Bio & PharmaSK Biopharmaceuticals gets marketing approval for epilepsy drug in Israel
Bio & PharmaSK Biopharmaceuticals gets marketing approval for epilepsy drug in IsraelMay 03, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharma's epilepsy treatment posts impressive growth in US
Bio & PharmaSK Biopharma's epilepsy treatment posts impressive growth in USNov 11, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharmaceuticals signs epilepsy drug deal with Brazil’s Eurofarma
Bio & PharmaSK Biopharmaceuticals signs epilepsy drug deal with Brazil’s EurofarmaJul 15, 2022 (Gmt+09:00)
2 Min read -
-
 PharmaceuticalsSK Biopharm aims to make it to global top 10 healthcare firms by 2030
PharmaceuticalsSK Biopharm aims to make it to global top 10 healthcare firms by 2030Jul 02, 2021 (Gmt+09:00)
1 Min read


