Bio & Pharma
Chong Kun Dang rallies on $1.3 bn deal with Novartis
This is the biggest-ever out-licensing deal the major S.Korean pharmaceutical company has ever signed
By Nov 06, 2023 (Gmt+09:00)
2
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund



Chong Kun Dang Pharmaceutical Corp.'s shares zoomed more than 25% on Monday on news that the South Korean medicine developer has signed its biggest-ever $1.3 billion out-licensing deal with Novartis AG for a Charcot-Marie-Tooth disease (CMT) treatment.
The company announced on Monday that it has signed a $1.3 billion licensing agreement with Novartis to grant the Swiss multinational pharmaceutical company the rights to develop and commercialize the Korean drugmaker’s CKD-510, an investigational drug for the rare hereditary disease CMT, also known as hereditary motor and sensory neuropathy (HMSN) or peroneal muscular atrophy (PMA).
Under the deal, Novartis will pay Chong Kun Dang an upfront fee of $80 million, milestone fees of $1.2 billion over time per the drug’s development and approval stages, as well as separate royalties for its sale.
This is the largest-ever out-licensing deal for the Korean pharmaceutical company since its inception more than eight decades ago, as well as this year's largest Korean pharmaceutical industry deal.
Chong Kun Dang's stock soared 26.1% to end at 128,000 won ($98.54) on Monday.
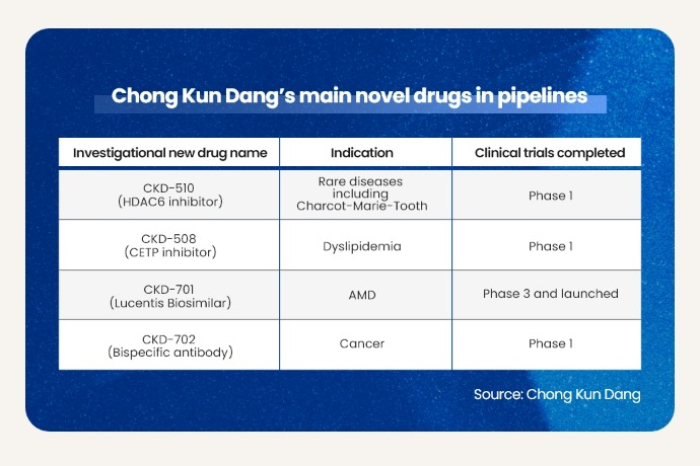
CKD-510 was developed by Chong Kun Dang as an inhibitor of histone deacetylase 6, or HDAC6, that regulates various inflammatory diseases.
ORPHAN DRUG FOR CMT
The Korean medicine developer proved the efficacy of CKD-510 in treating cardiovascular diseases and other HDAC6-related disorders during preclinical trials, as well as its safety and tolerability in Phase 1 trials in France.
CMT is known to damage the peripheral nerves, making it difficult to walk or perform normal tasks due to muscle atrophy in the hands and feet and loss of motor and sensory functions.
While there is still no fully authorized treatment for the hereditary disorder, the US Food and Drug Administration in March 2020 designated CKD-510 as an orphan drug to treat CMT.
Orphan status is granted to an investigative medicine for a rare disease, and orphan drug designation gives its developer incentives such as tax credits for qualified clinical trials and exemption from user fees in the US.
Chong Kun Dang plans to develop various treatments using the HDAC6 platform, the company said.
Under the latest deal, Novartis has been granted exclusive rights to develop and market CKD-510 in overseas markets except Korea, where Chong Kun Dang has the rights.
The Korean drug developer has spent about 12% of its annual revenue on average every year to develop breakthrough therapies or first-in-class drugs using novel mechanisms, said Kim Young-joo, the chief executive officer and chairman of Chong Kun Dang Pharmaceutical.
The latest development indicates that the Korean medicine developer has been successful in diversifying its medicine lineup beyond biologics and incrementally modified drugs to include innovative medicines.
It has about 10 new chemical entity (NCE) and biologics drugs in the pipeline, including CDK-510, according to the company.
Write to Ji-Hyun Lee at bluesky@hankyung.com
Sookyung Seo edited this article.
More to Read
-
 Bio & PharmaChong Kun Dang, Synaffix ink deal to introduce ADC technology
Bio & PharmaChong Kun Dang, Synaffix ink deal to introduce ADC technologyFeb 06, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaYbrain, Chong Kun Dang join forces for digital medicine distribution
Bio & PharmaYbrain, Chong Kun Dang join forces for digital medicine distributionJan 31, 2023 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


