Bio & Pharma
Samsung Bioepis gets FDA OK for its interchangeable biosimilar
Byooviz, a substitute for the macular degeneration medicine Lucentis, is S.Korea’s first to get such approval from America
By Oct 25, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


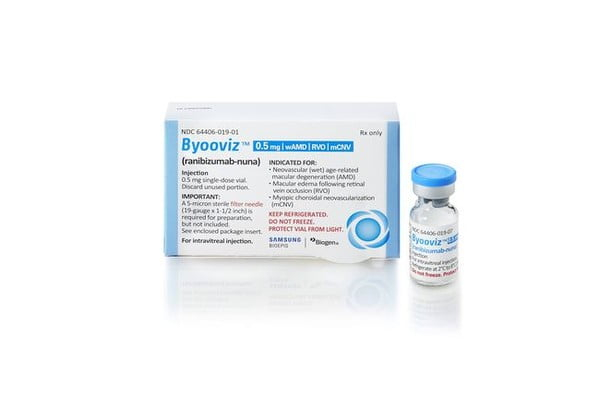
Samsung Bioepis is thus South Korea’s first pharmaceutical company to receive FDA designation for an interchangeable biosimilar.
Samsung Bioepis on Wednesday said it received this month official FDA approval for Byooviz’s upgraded status.
Use to treat macular degeneration, the original medicine Lucentis was developed by Roche (formerly Genentech) and is sold by the latter and Novartis. Of the treatment’s global sales last year of 3.8 trillion won ($2.8 billion), around 37% came from the US.
Officially launched in the US in June last year through the Cambridge, Massachusetts-based biotech company Biogen, Byooviz generated sales of 5.64 billion won ($4.3 million) in the second half of last year alone.
In South Korea, Samil Pharmaceutical Co., Ltd. secured the biosimilar’s distribution and sales rights and launched it in January this year under the name Amelivu.
Official recognition as an interchangeable biosimilar allows the latter’s prescription instead of the original drug at pharmacies, thus high competitiveness is expected for Byooviz on the US biosimilar market.
“This doesn’t mean that designated interchangeable biosimilars are different or superior in safety and effectiveness to regular biosimilars,” an industry source said. “But doctors and pharmacists reportedly feel less stress over giving alternative prescriptions for biosimilars recognized as interchangeable.”
Write to Ye-Na Kim at yena@hankyung.com
More to Read
-
 Bio & PharmaSamsung Bioepis releases Soliris biosimilar in Europe
Bio & PharmaSamsung Bioepis releases Soliris biosimilar in EuropeOct 19, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSamsung Bioepis to cooperate with Swiss firm Sandoz
Bio & PharmaSamsung Bioepis to cooperate with Swiss firm SandozSep 11, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSamsung Bioepis seeks to buy Biogen biosimilar unit
Bio & PharmaSamsung Bioepis seeks to buy Biogen biosimilar unitAug 02, 2023 (Gmt+09:00)
2 Min read -
 Bio & PharmaSamsung Bioepis enters US market for biosimilars of Humira
Bio & PharmaSamsung Bioepis enters US market for biosimilars of HumiraJul 14, 2023 (Gmt+09:00)
2 Min read -
 Bio & PharmaSamsung Bioepis to enhance biosimilar presence with Epysqli
Bio & PharmaSamsung Bioepis to enhance biosimilar presence with EpysqliJun 12, 2023 (Gmt+09:00)
3 Min read
Comment 0
LOG IN


