Bio & Pharma
GI Innovation seeks to license allergy therapy to Japan in 2023
The candidate targets diseases like chronic urticaria; the biotech is also accelerating clinical studies for immuno-oncology therapies
By Jul 17, 2023 (Gmt+09:00)
2
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


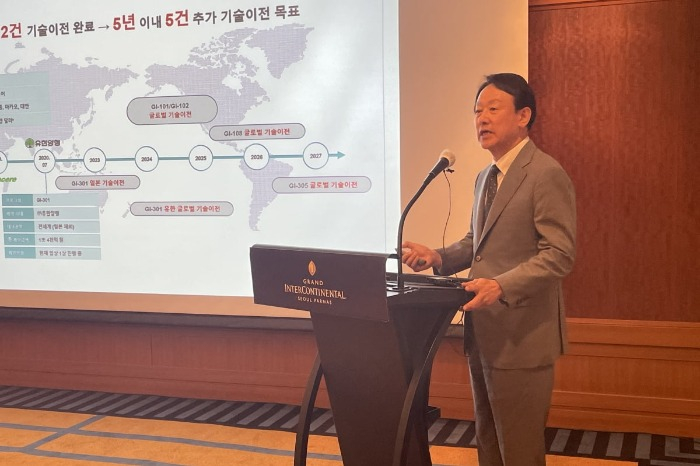
South Korea’s GI Innovation Inc. aims to complete licensing of its allergic diseases treatment candidate to a Japanese drug company by year's end, the Kosdaq-listed drug developer said at a press conference Monday in Seoul.
The drug candidate, GI-301, is a long-acting biologic that targets diseases such as chronic urticaria, atopic dermatitis and food allergies. GI Innovation licensed it out to Korea’s leading pharmaceutical company, Yuhan Corp., in 2020 for a 20 billion won ($15.8 million) upfront payment, with 89 billion won in milestone achievements and 1.3 trillion won in royalties for future sales.
GI Biome, the biotech’s affiliate, Yuhan Corp. and a global drug company are in talks over the technology transfer and negotiations have markedly progressed, said GI Innovation Chairman and CEO Rhee Byung-geon.
“Many local biotechs are seeking technology transfers. But I believe that is only an intermediate step, and true success is to commercialize the drugs and receive royalties on sales. We will achieve that,” said Lee.
GI Innovation will dispatch clinical trial experts to whichever company it will enter into a licensing agreement with, to prevent delays in the clinical research process, Lee added.
GI Innovation also introduced GI-108, a cancer immunotherapy candidate, under its pipeline.
The company has completed drug manufacturing for GI-108 clinical trials via a partnership with Samsung Biologics Co., the world’s top contract drugmaker by production capacity, and will file a clinical research application next year, said Executive Vice President for translational research Koh Young-jun.
GI-108 outperforms AstraZeneca plc’s Oleclumab in terms of inhibiting the CD73 enzyme, which is highly expressed in certain cancer cells. It also restricts tumor cell growth better than Merck & Co.’s Keytruda does, Koh added.
GI Innovation is now eyeing technology transfers of two other immune-oncology therapy candidates: GI-101 and GI-102.
The biotech is conducting clinical trial phase I and II for a modified GI-101, which has been found to increase the number of immune cells more than threefold compared with the previous version in animal tests.
GI-102 is a therapeutic candidate for use in cancer patients with insufficient immune cells, where immuno-oncology drugs are less effective. GI-102 is under clinical study in Korea and is set to begin overseas clinical research.
Write to Jeong Min Nam at peux@hankyung.com
Jihyun Kim edited this article.
More to Read
-
 Bio & PharmaBIO USA 2023 to draw S.Korean biotech leaders, startups
Bio & PharmaBIO USA 2023 to draw S.Korean biotech leaders, startupsMay 30, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSamsung to nurture biotech as next growth engine after chips: Lee
Bio & PharmaSamsung to nurture biotech as next growth engine after chips: LeeMay 08, 2023 (Gmt+09:00)
3 Min read -
 IPOsS.Korean biotech GI Innovation's IPO draws tepid market interest
IPOsS.Korean biotech GI Innovation's IPO draws tepid market interestMar 20, 2023 (Gmt+09:00)
2 Min read -
 Bio & PharmaKorean biopharma, biotech companies scale up as sales balloon
Bio & PharmaKorean biopharma, biotech companies scale up as sales balloonFeb 07, 2023 (Gmt+09:00)
3 Min read -
 Bio & PharmaSouth Korean biotech firms tap Middle East as next key market
Bio & PharmaSouth Korean biotech firms tap Middle East as next key marketFeb 06, 2023 (Gmt+09:00)
2 Min read
Comment 0
LOG IN


