Bio & Pharma
SK Biopharmaceuticals gets OK to sell epilepsy drug in Canada
Cenobamate is hailed for its excellent efficacy among third-generation medicines of its kind
By Jul 12, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator


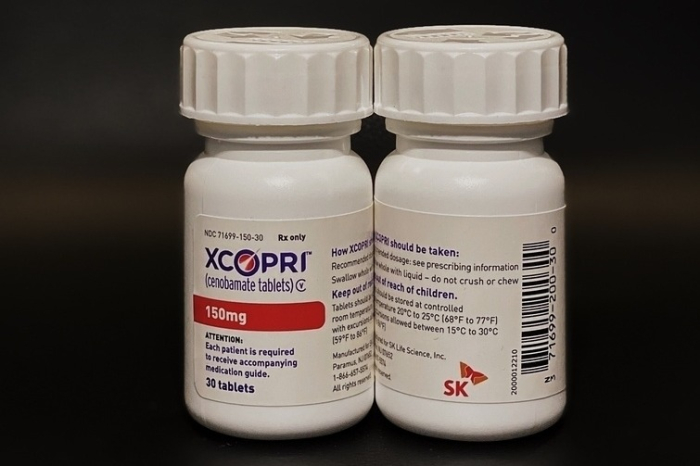
South Korea's SK Biopharmaceuticals has received approval in Canada to sell its new epilepsy drug Cenobamate under the product name Xcopri.
Having entered the US, the world's largest pharmaceutical market, in 2020, the company now seeks to expand sales in Canada, whose market globally ranks 10th.
SK Biopharmaceuticals last month received the OK to sell Cenobamate, which treats partial seizures in adults, from the federal agency Health Canada. Epilepsy is a brain disorder in which neurons turn abnormal and cause a sudden burst of electrical activity that causes a seizure.
SK Biopharmaceuticals is South Korea's lone company in the field to develop new drugs for the central nervous system, with two of its innovative drugs approved by the US Food and Drug Administration. As the latest new treatment for partial seizures to gain approval, Cenobamate is considered the most effective among third-generation epilepsy drugs.
SK Biopharmaceuticals is seen to accelerate its advance into North America, which accounts for over half of the global market for epilepsy treatment, through its entry into Canada, a top 10 pharmaceutical market. As of 2019, the world market for epilepsy treatment was worth $6.1 billion.
High profitability from the direct sale of Cenobamate is expected to boost SK Pharmaceuticals' earnings. In the US alone, sales of the medicine last year reached 169.2 billion won and in this year's first quarter, the figure grew 70% to 53.9 billion won year on year.
The company expects annual sales in America this year of between 270 billion and 300 billion won, up more than 60% from last year.
"We expect a return to the black in the fourth quarter," an SK Pharmaceuticals source said.
Turnover is also expected to rise in Europe. With its products sold in 18 European states including France, Germany and Belgium, the company will launch in four more countries there by the second half through its European sales partner Angelini Pharma.
Write to Yoo-Rim Kim at youforest@hankyung.com
More to Read
-
 Bio & PharmaSK Biopharmaceuticals gets marketing approval for epilepsy drug in Israel
Bio & PharmaSK Biopharmaceuticals gets marketing approval for epilepsy drug in IsraelMay 03, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharmaceuticals launches epilepsy drug in France
Bio & PharmaSK Biopharmaceuticals launches epilepsy drug in FranceDec 09, 2022 (Gmt+09:00)
1 Min read -

-
 Bio & PharmaSK Biopharmaceuticals signs epilepsy drug deal with Brazil’s Eurofarma
Bio & PharmaSK Biopharmaceuticals signs epilepsy drug deal with Brazil’s EurofarmaJul 15, 2022 (Gmt+09:00)
2 Min read
Comment 0
LOG IN


