Bio & Pharma
SK Biopharmaceuticals gets marketing approval for epilepsy drug in Israel
The S.Korean pharmaceutical company plans to expand Cenobamate into West Asian markets
By May 03, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


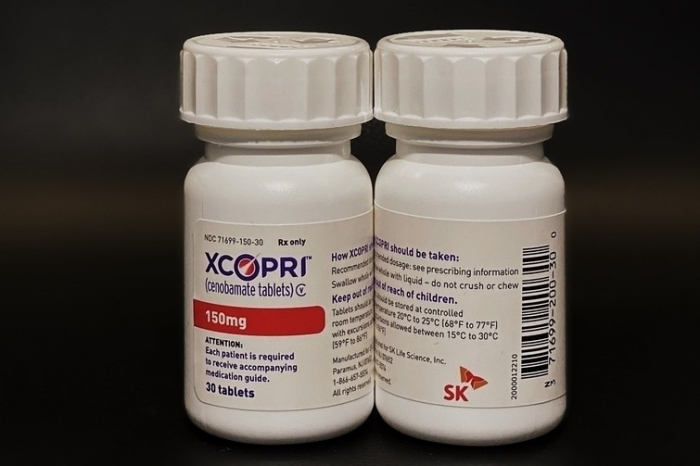
South Korea’s SK Biopharmaceuticals said on Wednesday that the Israeli Ministry of Health has approved the company's new drug application (NDA) for its epilepsy medication Cenobamate (local name Ecopri) on April 30.
The application was submitted by Dexcel Pharma, the Israeli partner of SK Biopharmaceuticals. The approved drug includes a total of six dosages: 12.5 mg, 25 mg, 50 mg, 100 mg, 150 mg and 200 mg.
Cenobamate, an independently developed epilepsy drug by SK Biopharmaceuticals, was recognized for its efficacy in treating partial-onset seizures in adults and received marketing approval from the US Food and Drug Administration (FDA) in November 2019.
Following the recent approval, SK Biopharmaceuticals stated plans to accelerate its market strategy in Western Asia, including Israel.
Write to Yoo-Rim Kim at youfrest@hankyung.com
More to Read
-
 Bio & PharmaSK Biopharm holds sales meeting for marketing Cenobamate in US
Bio & PharmaSK Biopharm holds sales meeting for marketing Cenobamate in USFeb 16, 2023 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharmaceuticals launches epilepsy drug in France
Bio & PharmaSK Biopharmaceuticals launches epilepsy drug in FranceDec 09, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharma's epilepsy treatment posts impressive growth in US
Bio & PharmaSK Biopharma's epilepsy treatment posts impressive growth in USNov 11, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaSK Biopharmaceuticals signs epilepsy drug deal with Brazil’s Eurofarma
Bio & PharmaSK Biopharmaceuticals signs epilepsy drug deal with Brazil’s EurofarmaJul 15, 2022 (Gmt+09:00)
2 Min read -
Comment 0
LOG IN


