Bio & Pharma
SK Bioscience's SKYCellflu approved by Chilean health authority
SKYCellflu has a shorter manufacturing period than traditional egg-based vaccines and has shown acceptable levels of immunogenicity in clinical trials
By Feb 02, 2023 (Gmt+09:00)
1
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


KT&G eyes overseas M&A after rejecting activist fund's offer


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


StockX in merger talks with Naver’s online reseller Kream


Meritz backs half of ex-manager’s $210 mn hedge fund


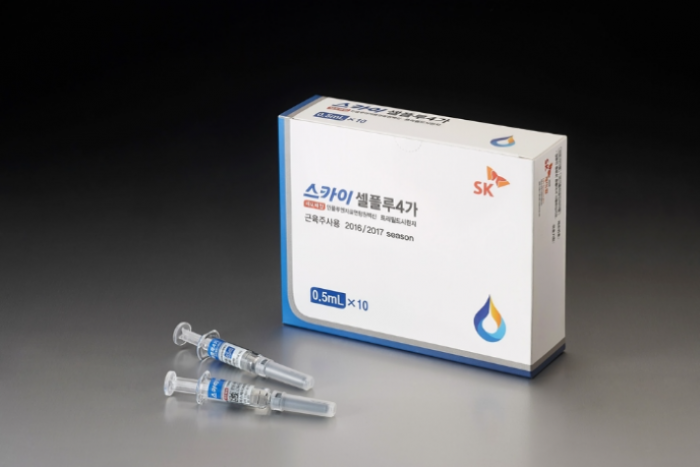
SK Bioscience Co., a bio and pharmaceutical affiliate of South Korea's SK Group, announced on Thursday that its world-first cell culture-based quadrivalent influenza vaccine SKYCellflu has received approval from the Instituto de Salud Publica de Chile (Chilean Institute of Public Health).
The company had previously obtained permission in Southeast Asia and the Middle East, including countries such as Malaysia and Thailand.
SK Bioscience plans to expand its global flu vaccine market into Central and South America with approval in Chile.
SKYCellflu has a shorter manufacturing period than traditional egg-based vaccines and has shown acceptable levels of immunogenicity and safety in clinical trials with over 1,500 adults and 453 children. The vaccine has also received pre-qualification from the World Health Organization.
Due to the COVID-19 pandemic, SK Bioscience temporarily suspended the manufacturing of SKYCellflu, but it will resume this year for the flu vaccination season.
"We will do our best to contribute public health beyond Korea to the world," said Ahn Jaeyong, CEO of SK Bioscience.
Write to Jung-Eun Kim at likesmile@hankyung.com
More to Read
-
 Bio & PharmaSK Bioscience's shingles vaccine obtains product approval from Malaysia
Bio & PharmaSK Bioscience's shingles vaccine obtains product approval from MalaysiaJan 09, 2023 (Gmt+09:00)
1 Min read -
 COVID-19SK Bioscience seeks WHO nod for prompt COVID vaccine sale
COVID-19SK Bioscience seeks WHO nod for prompt COVID vaccine saleSep 08, 2022 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


