Bio & Pharma
CENYX Biotech, developing treatment for brain damage with ROS-scavenging, nano-sized heavy metals
By Dec 26, 2022 (Gmt+09:00)
7
Min read
Most Read
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator



When severe brain diseases such as cerebral infarction or cerebral hemorrhage occur, the blood-brain barrier (BBB) in our body no longer functions properly.
It is because the brain has already been damaged to a large extent, and BBB barrier is also loosened. CENYX Biotech is developing a treatment for such severe brain injuries.
According to the company, the drug under development does not need to penetrate the BBB to treat the disease, which enables the company to focus more on enhancing efficacy and achieving a competitive edge.
Reactive oxygen species (ROS) are oxygen-containing molecules that are unstable due to the unpaired electrons existing on their outer shell.
As ROS are prone to exchanging unpaired electrons with other molecules for stabilization, they end up disturbing molecules in a stable state.
As a result, inflammation, aging, cancer, and degenerative diseases occur. Despite the harmful effects of ROS, no treatment based on this ROS-scavenging mechanism has been developed yet.
CENYX Biotech is aiming to develop the world's 1st ROS scavenging drug with a material called cerium oxide (CeO2).
“Our drugs target acute and severe diseases with significant unmet medical needs. We will continue to expand the scope of research regarding our pipeline to grow into a global nanomedicine company,” said Dr. Seung-Hoon Lee, CEO of CENYX Biotech.
THE WORLD'S FIRST CERIUM OXIDE(CeO2) NANOZYME DRUG IN DEVELOPMENT TO TACKLE BRAIN DISEASES
Cerium (Ce) is a ductile and iron-grey metal. The most stable state of cerium in nature is cerium oxide.
Due to its strong antioxidant property the material is widely used in industry such as an oil film remover for cars. The material is not expensive to get either.
Many researchers who recognized the material’s potential given its property and use tried to develop therapeutics using cerium oxide.
However, the big hurdle was safety. There were cases of workers developed pulmonary toxicity due to inhaled cerium oxide.
Cerium oxide is not ionized in the body and remains as a lump, damaging the delicate lung cells. Accordingly, attempts were made to make particles as small as nanometers.
Then, in 2006, a study result that demonstrated therapeutic efficacy of cerium oxide was published in an international journal for the first time in the world. The paper which showed the efficacy of cerium oxide in retinal degeneration was published in Nature Nanotechnology.
The second paper on the efficacy of cerium oxide nanoparticles came out in 2012. It was a study by CENYX Biotech that demonstrated the materials’ efficacy in reducing sterile inflammation that causes cerebral infarction.
Based on this result, CENYX Biotech has been developing CX201 based on cerium oxide nanozyme to treat brain diseases. The targeted indications are severe cerebral infarction and traumatic brain injury.
Nanozyme is a portmanteau of nanometer and enzyme. It refers to nanoparticles that act as enzymes in the body. When particles are broken down into fine nanometer-sized ones, the surface area to volume is maximized, which amplifies the enzymatic reaction . Therefore, only a small amount of drug can have a great therapeutic effect.
In addition to surgery, conservative treatment such as hemostasis and intracranial pressure management is the main treatment for brain damage such as cerebral hemorrhage worldwide. Currently, there is no treatment that directly targets the mechanism of damage, such as inflammation.
Dr. Lee expects that CX201 can be a new treatment that protects brain by suppressing oxidative stress with its strong antioxidant effect.
When cerium oxide enters the body, Ce3+ and Ce4+ coexist and oxygen vacancies are created on the surface. CX201 is a therapeutic agent based on the mechanism by which ROS interacts with this vacancy and is converted into water and oxygen and then disappears.
In order to safely and stably introduce water-immiscible cerium oxide into the body, it is coated with a proprietary polymer. This substance acts like a surfactant.
The efficacy of CX201 was demonstrated in an ongoing double-blind preclinical trial.
“When CX201 was intravenously administered once to an animal model of traumatic brain injury, the speed of neurological recovery was faster than that of the control group," said Dr. Lee.
FILING PHASE 1 IND APPLICATIONS FOR 2 PIPELINE ASSETS EXPECTED IN 2023
A treatment for subarachnoid hemorrhage is also being developed with this substance, which is CX213. Subarachnoid hemorrhage is a condition in which blood spreads in the brain due to ruptured cerebral aneurysm.
Compression or an acute inflammatory reaction may occur, leading to death. At this time, anti-inflammatory drugs such as steroids are usually administered, but this method that involves adaptive immunity reacts slowly, which can cause other complications during this time of reaction. .
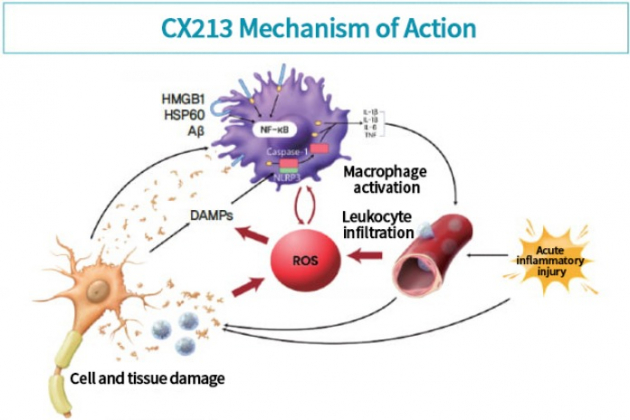
To prevent this, early emergency treatment is important. The only emergency rescue drug currently used is a clot-dissolving agent, ‘tissue plasminogen activator (tPA)’. However, tPA also has a limitation in that side effects such as bleeding and death increase when administered from 3 hours after the onset.
Mitsubishi Tanabe Japan developed Edaravone, an emergency rescue drug used for stroke rehabilitation and treatment of Lou Gehrig’s disease (ALS), but its utilization is poor. AstraZeneca in-licensed NXY-059 from Renovis and conducted clinical trials but failed to confirm the efficacy.
“In order to scavenge ROS which are generated as part of the reaction from the innate immunity, , an intense and potent therapeutic effect is required,” said Dr. Lee.
“CX213 will be able to increase the survival rate of patients with subarachnoid hemorrhage by scavenging ROS,” he added.
CX213 showed a survival rate more than three times higher than that of the control group in a randomized, investigator-blinded study of SAH animal model.
CENYX Biotech applied for the international Patent Cooperation Treaty (PCT) for CX213-based material patents in May 2021. In addition, it has established a mass production system for CX213 through a Contract Development and Manufacturing Organization (CDMO) in the United States and is conducting non-clinical trials with several global Clinical Research Organizations (CROs).
CENYX Biotech moved its headquarters to a building Seongsu-dong, Seoul with a size of about 480 square meters in June. The company’s research center is also located on the 4th floor of this building. In this research center, they plan to establish a pharmaceutical manufacturing and Good Manufacturing Practice (GMP) manufacturing facility.
The company plans to complete preclinical trials for its two pipeline assets by the end of next year. Phase 1 clinical trials will begin in 2024 at the latest, which will be conducted in the U.S. and Australia according to the company’s plan.
“The governments of Australia and New Zealand are providing active support to foreign companies conducting clinical trials in their countries, so we expect that we will be able to conduct fast clinical trials in these countries. After phase 1 in Australia, phase 2 will be conducted in the U.S,” said Dr. Lee.
The company has also initiated out-licensing activities. Dr. Lee participated in Bio Europe Fall 2022 in October and met business development and R&D professionals at multinational pharmaceutical companies.
"We initiated discussions with a number of global pharmaceutical companies including Merck (MSD), Johnson & Johnson, and Ipsen, and the discussion are still underway. People we met during Bio Europe Fall 2022 showed interests in our assets’ ROS scavenging properties and the high potential to receive orphan drug designation as a rescue therapy,” said Dr. Lee.
"After filing phase 1 Investigational New Drug (IND) applications for two assets at the end of next year, we expect to raise a round of Series B investments. We plan to go public in 2025 or 2026,” he added.
[COMMENTS BY EXPERTS]
CENYX Biotech's pipeline has high value. Appropriate measures are very important from the time of onset until surgical treatment, but there is no suitable drug for this indication. It is expected to be the 1st nanozyme-based new drug that improves the prognosis of many emergency patients with brain damage by minimizing secondary damage.(Joonsoo Kim, Sr. Team Leader at Partners Investment)
Write to Dohee Lee at tuxi0123@hankyung.com
More to Read
-
 Bio & PharmaLegoChem Bio, Amgen sign ADC platform deal for up to $1.2 bn
Bio & PharmaLegoChem Bio, Amgen sign ADC platform deal for up to $1.2 bnDec 23, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaToolGen, ANLBIO to jointly develop progeria treatments
Bio & PharmaToolGen, ANLBIO to jointly develop progeria treatmentsDec 21, 2022 (Gmt+09:00)
1 Min read -
 Bio & PharmaS.Korean biopharmaceutical sector to see brisk M&As in H1, 2023
Bio & PharmaS.Korean biopharmaceutical sector to see brisk M&As in H1, 2023Dec 21, 2022 (Gmt+09:00)
1 Min read
Comment 0
LOG IN


