SK invests in US digital healthcare firm Cala Health
SK Biopharmaceuticals is developing a digital therapeutic device for seizure detection and prediction
By May 19, 2022 (Gmt+09:00)
LG Chem to sell water filter business to Glenwood PE for $692 million


Kyobo Life poised to buy Japan’s SBI Group-owned savings bank


KT&G eyes overseas M&A after rejecting activist fund's offer


StockX in merger talks with Naver’s online reseller Kream


Mirae Asset to be named Korea Post’s core real estate fund operator



South Korea's SK Biopharmaceuticals Co. has dipped its toes into the rapidly growing digital healthcare market by investing in Cala Health, a US-based developer of treatments for neurological disorders.
SK Biopharmaceuticals and its parent company SK Inc. have jointly participated in Cala Health's Series D funding, in their first investment in a US digital healthcare company, SK said on Thursday.
“The investment in Cala Health will offer opportunities for potential technology collaboration with the US company in the new neurological space,” the South Korean company said.
The investment also paved the way for SK to make further strategic partnerships and investments in the US, the world's largest biotech and healthcare market, Cala Health said in a separate announcement on Wednesday, adding: “The investment further solidifies Cala as the leader of bioelectronic medicine."
Both companies did not disclose SK's investment amount.
Cala, headquartered in the San Francisco Bay Area, is known for its lead product of Cala TrioTM, a wearable device that reduces essential tremors and has been cleared by the US Food and Drug Administration.
It is now developing new treatments for Parkinson’s disease and other neurological disorders, as well as therapies for psychiatric and cardiology issues and autoimmune diseases.
Novartis International AG and Johnson & Johnson have also invested in the US company.
SK Biopharmaceuticals focuses on developing treatments for disorders of the central nervous system (CNS), for which it has two approved medicines and a pipeline of compounds in development, including epilepsy.
"As a strategic investor of Cala Health, we will cooperate in the field of neurology," said an SK official.
SK is currently working on a digital therapeutic device that can detect seizures for patients with epilepsy, which is expected to begin clinical trials at the end of this year. The new device under development will be previewed at the CES exhibition in Las Vegas next year.
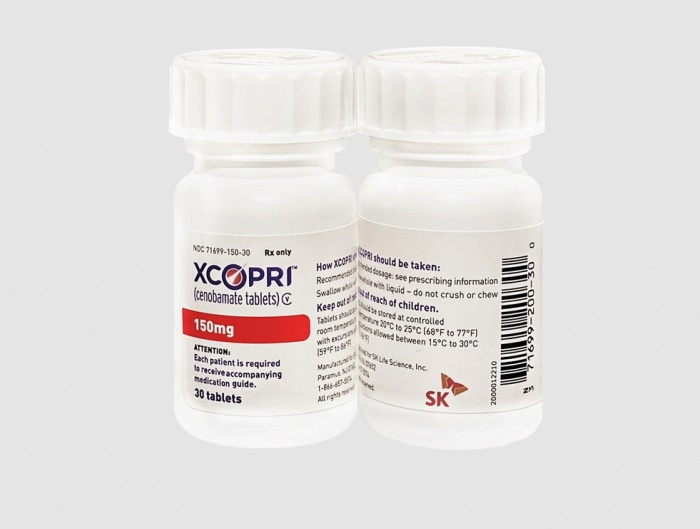
SK Pharmaceuticals has developed its epilepsy treatment Cenobamate. It is the first drug that a South Korean company has developed on its own from candidate substances discovery to clinical trials, licensing and sales in America
The bioelectronic medicine market is expected to grow at an annual average rate of 20.6% to $23.6 billion by 2030, according to Allied Market Research, a US-based research firm.
Write to Jae-Young Han at jyhan@hankyung.com
Yeonhee Kim edited this article.
-
-
 PharmaceuticalsSK Biopharm aims to make it to global top 10 healthcare firms by 2030
PharmaceuticalsSK Biopharm aims to make it to global top 10 healthcare firms by 2030Jul 02, 2021 (Gmt+09:00)
1 Min read


